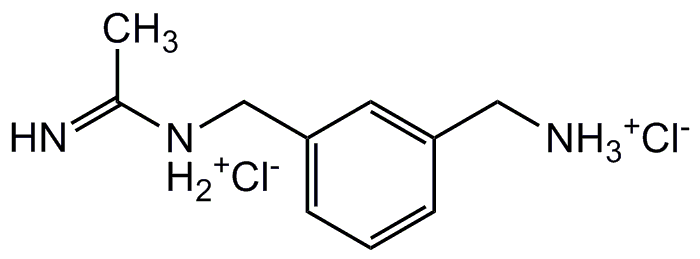
Chemical Structure
1400W . dihydrochloride [214358-33-5]

AG-CR1-0018
Estimated Purity>98% (NMR)
Product group Chemicals
Molecular Weight177.3 . 72.9
Overview
- SupplierAdipoGen Life Sciences
- Product Name1400W . dihydrochloride [214358-33-5]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>98% (NMR)
- Hazard InformationNon-hazardous
- Molecular FormulaC10H15N3 . 2HCl
- Molecular Weight177.3 . 72.9
- Scientific DescriptionA slow, tight binding and highly selective inhibitor of iNOS (inducible nitric oxide synthase/NOS II) (Kd < 7 nM) [1, 3, 4, 6]. Weak and reversible inhibition of nNOS (neuronal nitric oxide synthase/NOS I) (Ki < 2) and eNOS (endothelial nitric oxide synthase/NOS III) (Ki < 50 microM) [1]. Inhibits tumor growth [2]. Increases vasoconstriction to noradrenaline [7]. Improves contractile function [8]. Improves stroke outcome and decreases glutamate release [9]. Anti-inflammatory [10]. Significant AMPK activity suppressor. Review [5]. - Chemical. CAS: 214358-33-5. Formula: C10H15N3 . 2HCl. MW: 177.3 . 72.9. A slow, tight binding and highly selective inhibitor of iNOS (inducible nitric oxide synthase/NOS II) (Kd < 7 nM). Weak and reversible inhibition of nNOS (neuronal nitric oxide synthase/NOS I) (Ki < 2) and eNOS (endothelial nitric oxide synthase/NOS III) (Ki < 50 microM). Inhibits tumor growth. Increases vasoconstriction to noradrenaline. Improves contractile function. Improves stroke outcome and decreases glutamate release. Anti-inflammatory. Review.
- SMILES[Cl-].[Cl-].CC(=N)[NH2+]CC1=CC=CC(C[NH3+])=C1
- Storage Instruction2°C to 8°C
- UNSPSC12352200
References
- 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo: E.P. Garvey, et al.; J. Biol. Chem. 272, 4959 (1997)
- Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor: L.L. Thomsen, et al.; Cancer Res. 57, 3300 (1997)
- Actions of isoform-selective and non-selective nitric oxide synthase inhibitors on endotoxin-induced vascular leakage in rat colon: F. Laszlo & B.J.R. Whittle; Eur. J. Pharmacol. 334, 99 (1997)
- The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes: R. Boer, et al.; Mol. Pharmacol. 58, 1026 (2000)
- Nitric oxide synthases: structure, function and inhibition: W.K. Alderton, et al.; Biochem. J. 357, 593 (2001)
- Structural basis for the specificity of the nitric-oxide synthase inhibitors W1400 and Nomega-propyl-L-Arg for the inducible and neuronal isoforms: R. Fedorov, et al.; J. Biol. Chem. 278, 45818 (2003)
- Increased vasoconstriction to noradrenaline by 1400W, inhibitor of iNOS, in rats with streptozotocin-induced diabetes: X. Cheng & C.C. Pang; Eur. J. Pharmacol. 484, 263 (2004)
- Inhibition of iNOS with 1400W improves contractile function and alters nos gene and protein expression in reperfused skeletal muscle: P. Patel, et al.; Microsurgery 24, 324 (2004)
- Inhibition of iNOS activity by 1400W decreases glutamate release and ameliorates stroke outcome after experimental ischemia: F.J. Perez-Asensio, et al.; Neurobiol. Dis. 18, 375 (2005)
- Selective iNOS inhibitor 1400W enhances anti-catabolic IL-10 and reduces destructive MMP-10 in OA cartilage. Survey of the effects of 1400W on inflammatory mediators produced by OA cartilage as detected by protein antibody array: K. Järvinen, et al.; Clin. Exp. Rheumatol. 26, 275 (2008)
