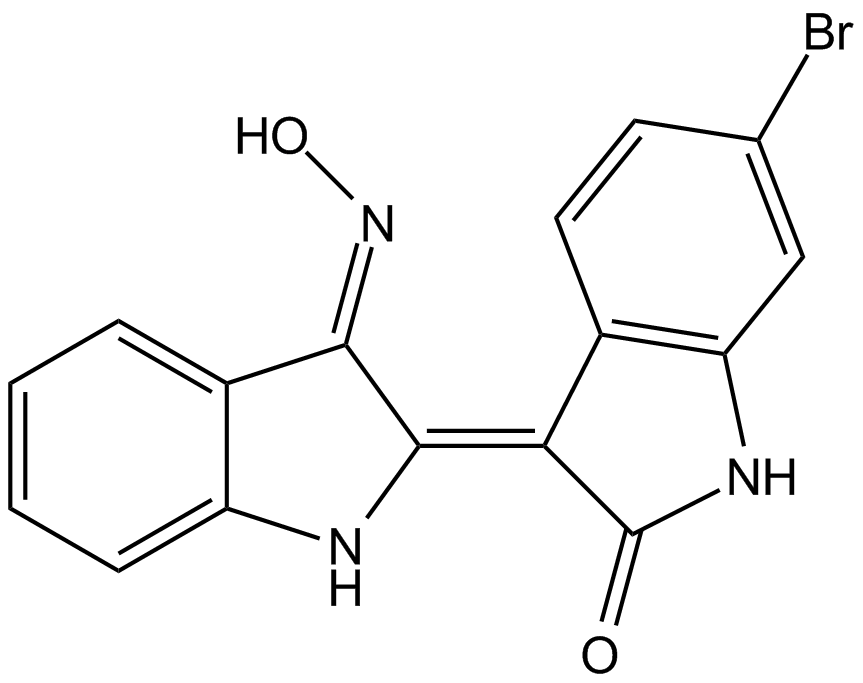
Chemical Structure
6BIO [667463-62-9]

AG-CR1-0056
CAS Number667463-62-9
Product group Chemicals
Estimated Purity>98% (NMR)
Molecular Weight356.2
Overview
- SupplierAdipoGen Life Sciences
- Product Name6BIO [667463-62-9]
- Delivery Days Customer10
- CAS Number667463-62-9
- CertificationResearch Use Only
- Estimated Purity>98% (NMR)
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC16H10BrN3O2
- Molecular Weight356.2
- Scientific DescriptionChemical. CAS: 667463-62-9. Formula: C16H10BrN3O2. MW: 356.2. Phosphoinositide-dependent kinase 1 (PDK1) inhibitor. Potent, reversible and ATP-competitive glycogen synthase kinase-3alpha/beta (GSK-3alpha/beta) inhibitor. JAK/STAT3 signaling inhibitor. Apoptosis inducer. Potent antiproliferative agent in malignant lymphoid cell. Antimetastatic agent. Potent chemosensitizing agent fighting TRAIL resistant cancer cells. - Phosphoinositide-dependent kinase 1 (PDK1) inhibitor. Potent, reversible and ATP-competitive glycogen synthase kinase-3alpha/beta (GSK-3alpha/beta) inhibitor. JAK/STAT3 signaling inhibitor. Apoptosis inducer. Potent antiproliferative agent in malignant lymphoid cell. Antimetastatic agent. Potent chemosensitizing agent fighting TRAIL resistant cancer cells.
- SMILESO\N=C1\C(\NC2=CC=CC=C\12)=C1\C(=O)NC2=C1C=CC(Br)=C2
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- GSK-3-selective inhibitors derived from Tyrian purple indirubins: L. Meijer, et al.; Chem. Biol. 10, 1255 (2003)
- Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases: P. Polychronopoulos, et al.; J. Med. Chem. 47, 935 (2004)
- The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes: A.S. Tseng, et al.; Chem. Biol. 13, 957 (2006)
- 7-Bromoindirubin-3'-oxime induces caspase-independent cell death: J. Ribas, et al.; Oncogene 25, 6304 (2006)
- Inverse in silico screening for identification of kinase inhibitor targets: S. Zahler, et al.; Chem. Biol. 14, 1207 (2007)
- An integrated computational approach to the phenomenon of potent and selective inhibition of aurora kinases B and C by a series of 7-substituted indirubins: V. Myrianthopoulos, et al.; J. Med. Chem. 50, 4027 (2007)
- Indirubin derivatives inhibit malignant lymphoid cell proliferation: A. Chebel, et al.; Leuk. Lymphoma 50, 2049 (2009)
- 6-Bromoindirubin-3'-Oxime Inhibits JAK/STAT3 Signaling and Induces Apoptosis of Human Melanoma Cells: L. Liu, et al.; Cancer Res. 71, 3972 (2011)
- Indirubin derivative 6BIO suppresses metastasis: S. Braig, et al.; Cancer Res. 73, 6004 (2013)
- The pleiotropic profile of the indirubin derivative 6BIO overcomes TRAIL resistance in cancer: S. Braig, et al.; Biochem. Pharmacol. 91, 157 (2014)
