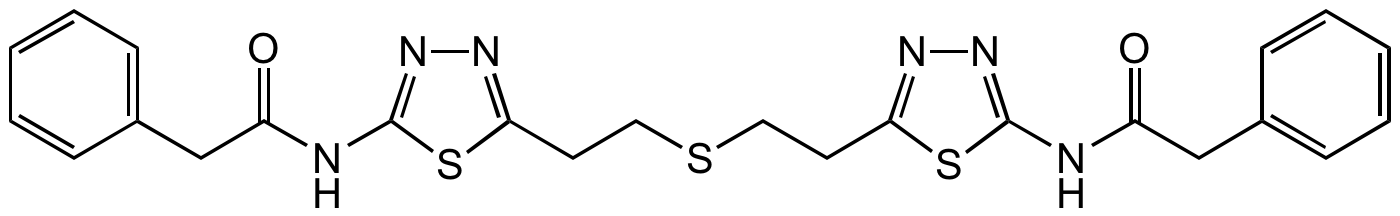
Chemical Structure
BPTES [314045-39-1]

AG-CR1-3690
CAS Number314045-39-1
Product group Chemicals
Estimated Purity>95%
Molecular Weight524.7
Overview
- SupplierAdipoGen Life Sciences
- Product NameBPTES [314045-39-1]
- Delivery Days Customer10
- CAS Number314045-39-1
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC24H24N6O2S3
- Molecular Weight524.7
- Scientific DescriptionChemical. CAS: 314045-39-1. Formula: C24H24N6O2S3. MW: 524.7. . Selective, allosteric non-competitive inhibitor of glutaminase 1 (GLS1), selective for GLS1 over GLS2, glutamate dehydrogenase, and gamma-glutamyl transpeptidase, consequently inhibiting glutaminolysis. Useful agent for immunometabolism research. Glutaminase converts glutamine to glutamate, which is an important excitatory neurotransmitter in brain and can be further oxidized to alpha-ketoglutarate to feed the tricarboxylic acid (TCA) cycle and to glutathione, which is important for controlling the level of reactive oxygen species (ROS), particularly important for cancer cell growth. Anticancer agent. Increases the production of reactive oxygen species and reduces ATP levels in hypoxic cells, induces cell death of P493 human lymphoma B cells in vitro and delays tumor xenograft growth in vivo. - Selective, allosteric non-competitive inhibitor of glutaminase 1 (GLS1), selective for GLS1 over GLS2, glutamate dehydrogenase, and gamma-glutamyl transpeptidase, consequently inhibiting glutaminolysis. Useful agent for immunometabolism research. Glutaminase converts glutamine to glutamate, which is an important excitatory neurotransmitter in brain and can be further oxidized to alpha-ketoglutarate to feed the tricarboxylic acid (TCA) cycle and to glutathione, which is important for controlling the level of reactive oxygen species (ROS), particularly important for cancer cell growth. Anticancer agent. Increases the production of reactive oxygen species and reduces ATP levels in hypoxic cells, induces cell death of P493 human lymphoma B cells in vitro and delays tumor xenograft growth in vivo.
- SMILESO=C(CC1=CC=CC=C1)NC2=NN=C(CCSCCC3=NN=C(NC(CC4=CC=CC=C4)=O)S3)S2
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES): M.M. Robinson, et al.; Biochem. J. 406, 407 (2007)
- Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1: M.J. Seltzer, et al.; Cancer Res. 70, 8981 (2010)
- Full-length human glutaminase in complex with an allosteric inhibitor: B. DeLaBarre, et al.; Biochemistry 50, 10764 (2011)
- BPTES inhibition of hGA(124-551), a truncated form of human kidney-type glutaminase: E.W. Hartwick & N.P. Curthoys; J. Enzyme Inhib. Med. Chem. 27, 861 (2012)
- Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells: A. Le, et al.; Cell Metab. 15, 110 (2012)
- Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors: K. Shukla, et al.; J. Med. Chem. 55, 10551 (2012)
- Availability of the key metabolic substrates dictates the respiratory response of cancer cells to the mitochondrial uncoupling: A.V. Zhdanov, et al.; Biochim. Biophys. Acta 1837, 51 (2014)
- Small molecule glutaminase inhibitors block glutamate release from stimulated microglia: A.G. Thomas, et al.; BBRC 443, 32 (2014)
- Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis: Y. Xiang, et al.; J. Clin. Invest. 125, 2293 (2015)
- Design and evaluation of novel glutaminase inhibitors: L.A. McDermott, et al.; Bioorg. Med. Chem. 24, 1819 (2016)
