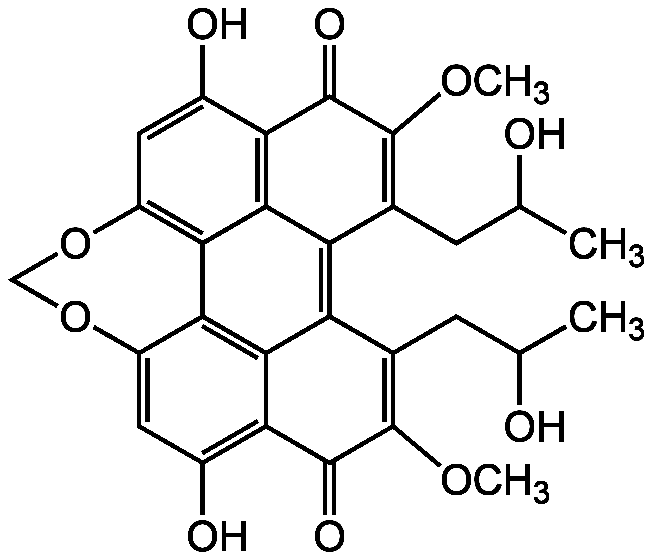
Chemical Structure
Cercosporin [35082-49-6]

AG-CN2-0111
CAS Number35082-49-6
Product group Chemicals
Estimated Purity>95% (HPLC)
Molecular Weight534.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameCercosporin [35082-49-6]
- Delivery Days Customer10
- CAS Number35082-49-6
- CertificationResearch Use Only
- Estimated Purity>95% (HPLC)
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC29H26O10
- Molecular Weight534.5
- Scientific DescriptionChemical. CAS: 35082-49-6. Formula: C29H26O10. MW: 534.5. Isolated from Cercospora sp. Phytotoxin. Potent and specific PKC inhibitor. Competes for the phorbol binding site. Anticancer compound. Cytotoxic. Antiproliferative. Antiparasitic. Antimicrobial. - Phytotoxin [1, 4]. Potent and specific PKC inhibitor. Competes for the phorbol binding site [2, 3, 6]. Anticancer compound. Cytotoxic [5, 6, 7]. Antiproliferative [5, 6]. Antiparasitic [7, 8]. Antimicrobial [8]. Photosensitizer, singlet oxygen 1O2 producer. Antimalaria
- SMILESCOC1=C(CC(C)O)C2=C3C(CC(C)O)=C(OC)C(=O)C4=C(O)C=C5OCOC6=C(C5=C34)C2=C(C(O)=C6)C1=O
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Light-induced production of singlet oxygen and superoxide by the fungal toxin, cercosporin: M.E. Daub & R.P. Hangarter; Plant Physiol. 73, 855 (1983)
- Calphostin (UCN1028) and calphostin related compounds, a new class of specific and potent inhibitors of protein kinase C: T. Tamaoki, et al.; Adv. Second Messenger Phosphoprotein Res. 24, 497 (1990)
- Structural investigation of protein kinase C inhibitors: D. Barak, et al.; J. Mol. Struct. 230, 419 (1991)
- Cell surface redox potential as a mechanism of defense against photosensitizers in fungi: C.C. Sollod, et al.; Appl. Environ. Microbiol. 58, 444 (1992)
- Cytotoxicity and antiproliferative effect of hypericin and derivatives after photosensitization: A.L. Vandenbogaerde, et al.; Photochem. Photobiol. 67, 119 (1998)
- Design, synthesis, and investigation of protein kinase C inhibitors: total syntheses of (+)-calphostin D, (+)-phleichrome, cercosporin, and new photoactive perylenequinones: B.J. Morgan, et al.; JACS 131, 9413 (2009)
- Chemical constituents of the new endophytic fungus Mycosphaerella sp. nov. and their anti-parasitic activity: E. Moreno, et al.; Nat. Prod. Commun. 6, 835 (2011)
- Antiprotozoal and antimicrobial compounds from the plant pathogen Septoria pistaciarum: M. Kumarihamy, et al.; J. Nat. Prod. 75, 883 (2012)
