Chemical Structure
Colchicine [64-86-8]

AG-CN2-0048
CAS Number64-86-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight399.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameColchicine [64-86-8]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number64-86-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC22H25NO6
- Molecular Weight399.4
- Scientific DescriptionAnti-cancer compound [5]. Microtubule assembly inhibitor. Depolymerizes microtubules and limits microtubule formation (inactivates spindle fibre formation) [1, 2, 5, 6]. Inhibits mitosis during cell division at metaphase by inhibiting spindle formation [6]. Anti-inflammatory compound [7, 9]. Suppresses monosodium urate crystal-induced NLRP3/NALP3 inflammasome-driven caspase-1 activation, IL-1beta processing and release, and L-selectin expression on neutrophils at micromolar concentrations. Blocks the release of a crystal-derived chemotactic factor from neutrophil lysosomes, blocks neutrophil adhesion to, and inhibits monosodium urate crystal-induced production of superoxide anions from neutrophils at nanomolar concentrations [7]. Drug used in treatment of gout, familial Mediterranean fever, pericarditis and Behects disease. Investigated for its anti-cancer activity [5, 8, 9]. It has a narrow therapeutic index with no clear-cut distinction between nontoxic, toxic and lethal doses, causing substantial confusion among clinicians [8]. Apoptosis inducer in a variety of normal and tumor cell lines [3, 4]. Inhibitor of autophagosome-lysosome fusion. Inhibits acetylated a-tubulin mediated dynein dependent transport of mitochondria and subsequent apposition of ASC on mitochondria to NLRP3 on the endoplasmic reticulum in vitro and in vivo. - Chemical. CAS: 64-86-8. Formula: C22H25NO6. MW: 399.4. Isolated from Colchicum autumnale. Anti-cancer compound. Microtubule assembly inhibitor. Depolymerizes microtubules and limits microtubule formation (inactivates spindle fibre formation). Inhibits mitosis during cell division at metaphase by inhibiting spindle formation. Anti-inflammatory compound. Suppresses monosodium urate crystal-induced NLRP3/NALP3 inflammasome-driven caspase-1 activation, IL-1beta processing and release, and L-selectin expression on neutrophils at micromolar concentrations. Blocks the release of a crystal-derived chemotactic factor from neutrophil lysosomes, blocks neutrophil adhesion to, and inhibits monosodium urate crystal-induced production of superoxide anions from neutrophils at nanomolar concentrations. Drug used in treatment of gout, familial Mediterranean fever, pericarditis and Behects disease. Investigated for its anti-cancer activity. It has a narrow therapeutic index with no clear-cut distinction between nontoxic, toxic and lethal doses, causing substantial confusion among clinicians. Apoptosis inducer in a variety of normal and tumor cell lines. Inhibitor of autophagosome-lysosome fusion. Inhibits acetylated a-tubulin mediated dynein dependent transport of mitochondria and subsequent apposition of ASC on mitochondria to NLRP3 on the endoplasmic reticulum in vitro and in vivo.
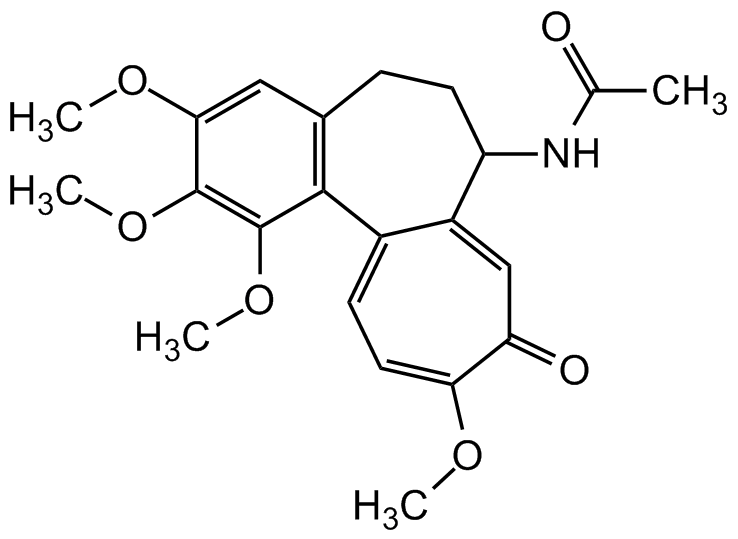
- SMILESCOC1=CC2=C(C(OC)=C1OC)C1=CC=C(OC)C(=O)C=C1C(CC2)NC(C)=O
- Storage Instruction2°C to 8°C,-20°C
- UN NumberUN 3462
- UNSPSC12352200
References
- Interactions of colchicine with tubulin: S.B. Hastie; Pharmacol. Ther. 51, 377 (1991) (Review)
- Analysis of the colchicine-binding site of beta-tubulin: R.G. Burns; FEBS Lett. 297, 205 (1992) (Review)
- Colchicine induces apoptosis in cerebellar granule cells: E. Bonfoco, et al.; Exp. Cell. Res. 218, 189 (1995)
- Sustained JNK activation induces endothelial apoptosis: studies with colchicine and shear stress: Y.L. Hu, et al.; Am. J. Physiol. 277, H1593 (1999)
- Microtubules as a target for anticancer drugs: M.A. Jordan & L. Wilson; Nat. Rev. Cancer 4, 253 (2004)
- Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin: B. Bhattacharyya, et al.; Med. Res. Rev. 28, 155 (2008) (Review)
- Colchicine: its mechanism of action and efficacy in crystal-induced inflammation: G. Nuki; Curr. Rheumatol. Rep. 10, 218 (2008) (Review)
- Colchicine poisoning: the dark side of an ancient drug: Y. Finkelstein, et al.; Clin. Toxicol. (Phila). 48, 407 (2010) (Review)
- Colchicine for the treatment of gout: P. Richette & T. Bardin; Expert Opin. Pharmacother. 11, 2933 (2010) (Review)
- Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome: T. Misawa, et al.; Nat. Immunol. 14, 454 (2013)
