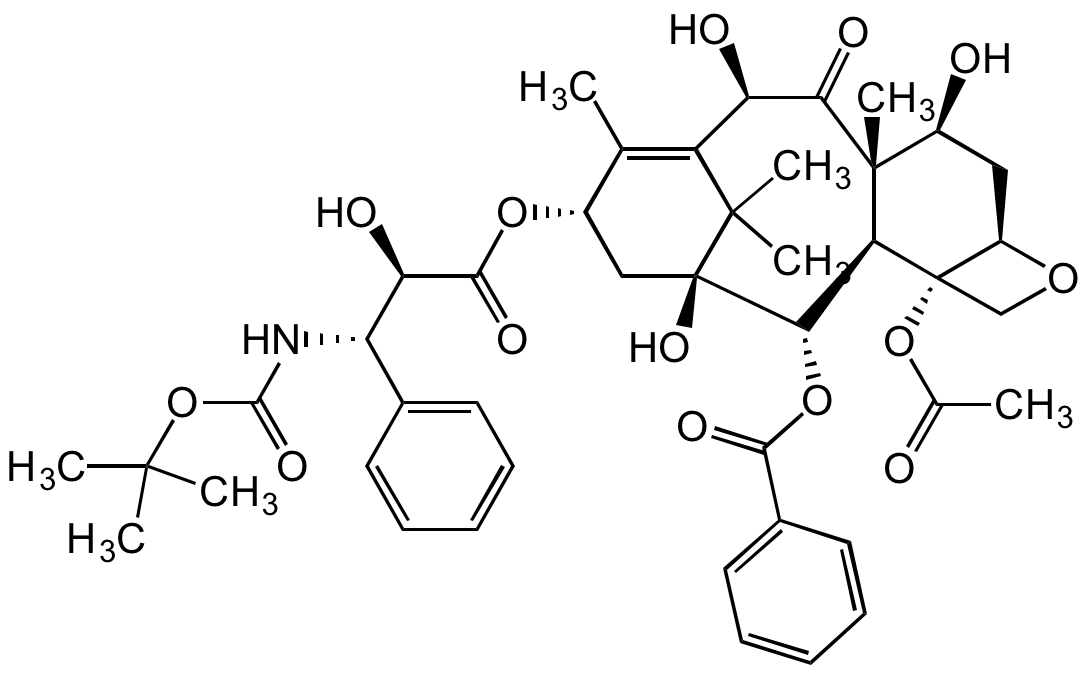
Chemical Structure
Docetaxel [114977-28-5]

CDX-D0371
CAS Number114977-28-5
Product group Chemicals
Estimated Purity>97%
Molecular Weight807.88
Overview
- SupplierChemodex
- Product NameDocetaxel [114977-28-5]
- Delivery Days Customer10
- CAS Number114977-28-5
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationNon-hazardous
- Molecular FormulaC43H53NO14
- Molecular Weight807.88
- Scientific DescriptionAnticancer agent. Semisynthetic analog of taxol (paclitaxel). Inhibits microtubule disassembly and inhibits cell replication. Binds to and stabilizes the beta-tubulin subunit of microtubules, preventing depolymerization of the mitotic spindle thus leading to cell cycle arrest at G2/M and apoptosis. This cytotoxic activity has been widely exploited in suppressing oncogenic proliferation. Displays potent and broad antineoplastic properties, used mainly for the treatment of breast, ovarian, and non-small cell lung cancer. Also inhibits pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and displays immunomodulatory and pro-inflammatory properties by inducing various mediators of the inflammatory response. Also studied for use as a radiation-sensitizing agent. - Chemical. CAS: 114977-28-5. Formula: C43H53NO14. MW: 807.88. Synthetic. Anticancer agent. Semisynthetic analog of taxol (paclitaxel). Inhibits microtubule disassembly and inhibits cell replication. Binds to and stabilizes the beta-tubulin subunit of microtubules, preventing depolymerization of the mitotic spindle thus leading to cell cycle arrest at G2/M and apoptosis. This cytotoxic activity has been widely exploited in suppressing oncogenic proliferation. Displays potent and broad antineoplastic properties, used mainly for the treatment of breast, ovarian, and non-small cell lung cancer. Also inhibits pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and displays immunomodulatory and pro-inflammatory properties by inducing various mediators of the inflammatory response. Also studied for use as a radiation-sensitizing agent.
- SMILESCC(C)(OC(N[C@H]([C@H](C(O[C@H]1C[C@@]([C@H]([C@@H]([C@@]2(C([C@@H](C3=C1C)O)=O)C)[C@]4([C@@H](C[C@@H]2O)OC4)OC(C)=O)OC(C5=CC=CC=C5)=O)(C3(C)C)O)=O)O)C6=CC=CC=C6)=O)C
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
