Chemical Structure
Glyburide (USP) [10238-21-8]

AG-CR1-3613
CAS Number10238-21-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight494
Overview
- SupplierAdipoGen Life Sciences
- Product NameGlyburide (USP) [10238-21-8]
- Delivery Days Customer10
- CAS Number10238-21-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous
- Molecular FormulaC23H28ClN3O5S
- Molecular Weight494
- Scientific DescriptionAntidiabetic compound. Binds to and activates the ATP-sensitive potassium channels (KATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1). Causes cell membrane depolarization and opening of voltage-dependent calcium channel. Increases intracellular calcium and stimulates insulin secretion in beta cells NLRP3 inflammasome inhibitor. Broad-spectrum ATP-binding cassette (ABC) transporter inhibitor. Shown to have anti-leishmanial activity. Potential inhibitor of collagenases. - Chemical. CAS: 10238-21-8. Formula: C23H28ClN3O5S. MW: 494. Antidiabetic compound. Binds to and activates the ATP-sensitive potassium channels (KATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1). Causes cell membrane depolarization and opening of voltage-dependent calcium channel. Increases intracellular calcium and stimulates insulin secretion in beta cells NLRP3 inflammasome inhibitor. Broad-spectrum ATP-binding cassette (ABC) transporter inhibitor. Shown to have anti-leishmanial activity. Potential inhibitor of collagenases.
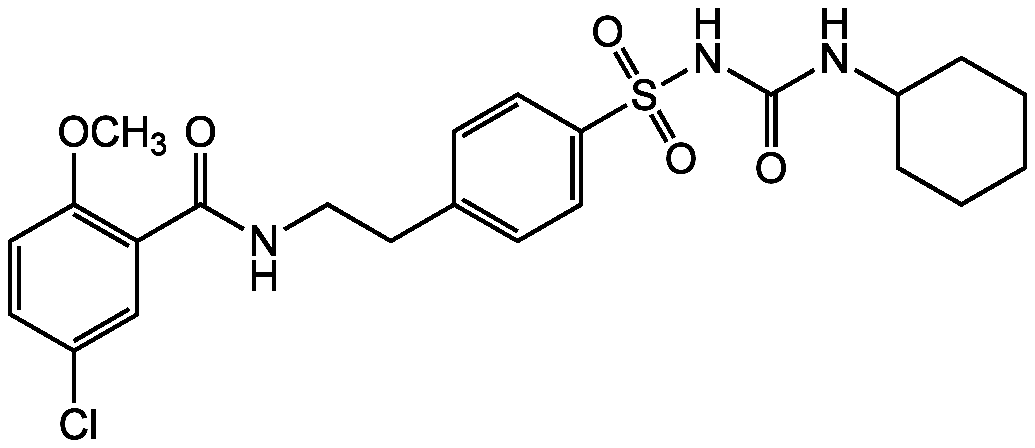
- SMILESCOC1=C(C=C(Cl)C=C1)C(=O)NCCC1=CC=C(C=C1)S(=O)(=O)NC(=O)NC1CCCCC1
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Glyburide: a second-generation sulfonylurea hypoglycemic agent. History, chemistry, metabolism, pharmacokinetics, clinical use and adverse effects: J.M. Feldman; Pharmacotherapy 5, 43 (1985)
- The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells: H. Schmid-Antomarchi, et al.; J. Biol. Chem. 262, 15840 (1987)
- Antidiabetic sulfonylureas control action potential properties in heart cells via high affinity receptors that are linked to ATP-dependent K+ channels: M. Fosset, et al.; J. Biol. Chem. 263, 7933 (1988)
- Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator: C.M. McNicholas, et al.; PNAS 93, 8083 (1996)
- The sulphonylurea glibenclamide inhibits multidrug resistance protein (MRP1) activity in human lung cancer cells: L. Payen, et al.; Br. J. Pharmacol. 132, 778 (2001)
- Glibenclamide, a blocker of K+(ATP) channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis: X. Serrano-Martin, et al.; Antimicrob. Agents Chemother. 50, 4214 (2006)
- CFTR inhibition by glibenclamide requires a positive charge in cytoplasmic loop three: P. Melin, et al.; Biochim. Biophys. Acta 1768, 2438 (2007)
- Glyburide inhibits the Cryopyrin/Nalp3 inflammasome: M. Lamkanfi, et al.; J. Cell. Biol. 187, 61 (2009)
- Glyburide in treating malignant cerebral edema. Blocking sulfonyl urea one (SUR1) receptors: T.V. Pallan & I. Ahmed; J. Vasc. Interv. Neurol. 7, 23 (2014)
- In vitro biological evaluation of glyburide as potential inhibitor of collagenases: V.L. Bodiga, et al.; Int. J. Biol. Macromol. 70, 187 (2014)
