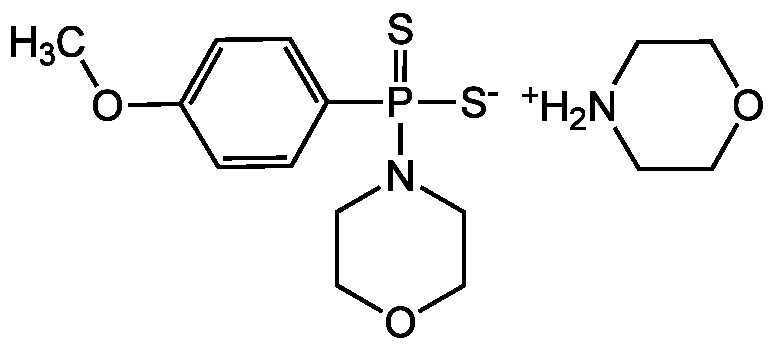
Chemical Structure
GYY 4137 [106740-09-4]

AG-CR1-3513
CAS Number106740-09-4
Product group Chemicals
Estimated Purity>95% (NMR)
Molecular Weight288.4 . 87.1
Overview
- SupplierAdipoGen Life Sciences
- Product NameGYY 4137 [106740-09-4]
- Delivery Days Customer10
- CAS Number106740-09-4
- CertificationResearch Use Only
- Estimated Purity>95% (NMR)
- Hazard InformationNon-hazardous
- Molecular FormulaC11H16NO2PS2 . C4H9NO
- Molecular Weight288.4 . 87.1
- Scientific DescriptionChemical. CAS: 106740-09-4. Formula: C11H16NO2PS2 . C4H9NO. MW: 288.4 . 87.1. Water-soluble, slow-releasing hydrogen sulfide (H2S) donor. Exhibits vasodilator and antihypertensive activity. Anti-inflammatory. Inhibits LPS-induced release of proinflammatory mediators (IL-1beta, IL-6, TNF-alpha, nitric oxide (NO) and PGE2) and increases the synthesis of the anti-inflammatory chemokine IL-10 through NF-kappaB, ATF-2 and HSP27 dependent pathways. Causes stomatal opening and reduces nitric oxide accumulation in plants. Oxidative stress-induced cell death inhibitor. Shows novel anti-cancer effects in vitro and in vivo. Anti-thrombotic via p-selectin dependent mechanism. - Water-soluble, slow-releasing hydrogen sulfide (H2S) donor [1]. Exhibits vasodilator and antihypertensive activity [1, 2, 4]. Anti-inflammatory [2, 3]. Inhibits LPS-induced release of proinflammatory mediators (IL-1beta, IL-6, TNF-alpha, nitric oxide (NO) and PGE2) and increases the synthesis of the anti-inflammatory chemokine IL-10 through NF-kappaB, ATF-2 and HSP27 dependent pathways [2, 3]. Causes stomatal opening and reduces nitric oxide accumulation in plants [5]. Oxidative stress-induced cell death inhibitor [6]. Shows novel anti-cancer effects in vitro and in vivo [7]. Anti-thrombotic via p-selectin dependent mechanism [8].
- SMILESC1COCC[NH2+]1.COC1=CC=C(C=C1)P([S-])(=S)N1CCOCC1
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide.: L. Li et, al.; Circulation 117, 2351 (2008)
- GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat.: L. Li, et al.; Free Radic. Biol. Med. 47, 103 (2009)
- The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages: M. Whiteman, et al.; Antioxid. Redox Signal. 12, 1147 (2010)
- Hydrogen sulfide and its modulation in arterial hypertension and atherosclerosis: J. Bełtowski, et al.; Cardiovasc. Hematol. Agents Med. Chem. 8, 173 (2010)
- A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation: M. Lisjak, et al.; Plant Physiol. Biochem. 48, 931 (2010)
- Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: is h(2) S a novel cytoprotective mediator in the inflamed joint?: B. Fox, et al.; J. Cell. Mol. Med. 16, 896 (2012)
- The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo: Z.W. Lee, et al.; PLOS One 6, e21077 (2011)
- The slow releasing H2S donor GYY 4137 exerts anti-thrombotic effects via a P-selectin dependent mechanism: E. Grambow, et al.; DGCH München (Meeting Abstract) (2011)
