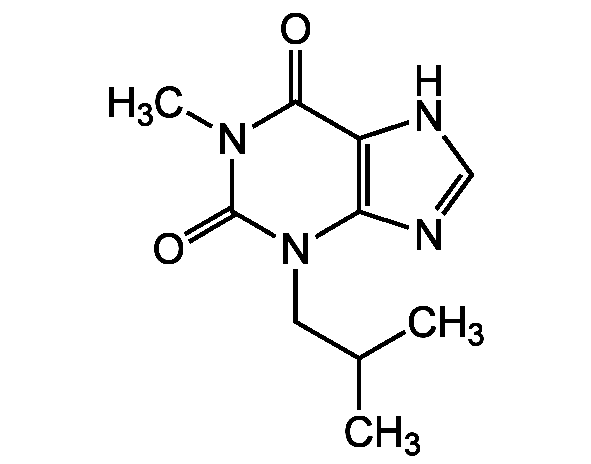
Chemical Structure
IBMX [28822-58-4]

AG-CR1-3512
CAS Number28822-58-4
Product group Chemicals
Estimated Purity>99%
Molecular Weight222.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameIBMX [28822-58-4]
- Delivery Days Customer10
- CAS Number28822-58-4
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC10H14N4O2
- Molecular Weight222.3
- Scientific DescriptionCell permeable, competitive, non-specific cAMP and cGMP phosphodiesterase inhibitor [1, 5, 6, 11]. Increases cAMP levels that activate PKA, leading to decreased proliferation, increased differentiation and induction of apoptosis [9]. Enhances differentiation of 3T3-L1 cells [3]. Non-selective adenosine receptor antagonist [4]. Inhibits Ca2+ ion channels [7, 8]. Activates TNF-alpha [10]. Adipogenic [10]. Activates leukotriene synthesis [12]. Reduces inflammation and innate immunity [12]. - Chemical. CAS: 28822-58-4. Formula: C10H14N4O2. MW: 222.3. Cell permeable, competitive, non-specific cAMP and cGMP phosphodiesterase inhibitor. Increases cAMP levels that activate PKA, leading to decreased proliferation, increased differentiation and induction of apoptosis. Enhances differentiation of 3T3-L1 cells. Non-selective adenosine receptor antagonist. Inhibits Ca2+ ion channels. Activates TNF-alpha. Adipogenic. Activates leukotriene synthesis. Reduces inflammation and innate immunity.
- SMILESCC(C)CN1C2=C(NC=N2)C(=O)N(C)C1=O
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Effects of xanthine derivatives on lipolysis and on adenosine 3',5'- monophosphate phosphodiesterase activity: J.A. Beavo, et al.; Mol. Pharmacol. 6, 597 (1970)
- Inhibition of growth of primary and metastatic Lewis lung carcinoma cells by the phosphodiesterase inhibitor isobutylmethylxanthine: P. Janik, et al.; Cancer Res. 40, 1950 (1980)
- A role for soluble cAMP phosphodiesterases in differentiation of 3T3-L1adipocytes: M.L. Elks & V.C. Manganiello; J. Cell Physiol. 124, 191 (1985)
- Adenosine receptors: development of selective agonists and antagonists: J.W. Daly, et al.; Prog. Clin. Biol. Res. 230, 41 (1987)
- Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors: J.A. Beavo & D.H. Reifsnyder; TIPS 11, 150 (1990)
- Isobutylmethylxanthine and other classical cyclic nucleotide phosphodiesterase inhibitors affect cAMP-dependent protein kinase activity: C. Tomes, et al.; Cell Signal. 5, 615 (1993)
- IBMX induces calcium release from intracellular stores in rat sensory neurones: Y. Usachev & A. Verkhratsky; Cell Calcium 17, 197 (1995)
- Inhibition of recombinant human cardiac L-type Ca2+ channel alpha1C subunits by 3-isobutyl-1-methylxanthine: I.M. Fearon, et al.; Eur. J. Pharmacol. 342, 353 (1998)
- Up-regulation of the cAMP/PKA pathway inhibits proliferation, induces differentiation, and leads to apoptosis in malignant gliomas: T.C. Chen, et al.; Lab. Invest. 78, 165 (1998)
- The phosphodiesterase inhibitor IBMX suppresses TNF-alpha expression in human adipocyte precursor cells: a possible explanation for its adipogenic effect: F. Hube, et al.; Horm. Metab. Res. 31, 359 (1999)
