Chemical Structure
Jasplakinolide (high purity) [102396-24-7]

AG-CN2-0037
Overview
- SupplierAdipoGen Life Sciences
- Product NameJasplakinolide (high purity) [102396-24-7]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous,Warning
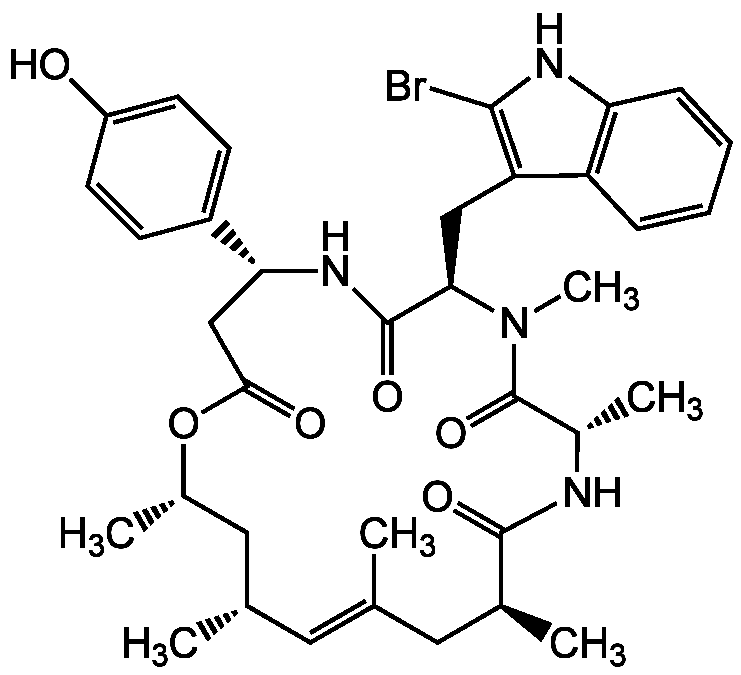
- Molecular FormulaC36H45BrN4O6
- Molecular Weight709.7
- Scientific DescriptionCell permeable, non-fluorescent F-actin probe [3, 8, 12]. Potent inducer of actin polymerization and stabilization [3, 8, 12]. Competes with phallotoxins for actin binding [3]. Antifungal and antiparasitic compound [1, 2, 9]. Antiproliferative and anticancer compound [3, 4, 5]. Apoptosis enhancer/inducer [6, 10]. Tool used for autophagy/phagocytosis research [7, 11, 13]. - Chemical. CAS: 102396-24-7. Formula: C36H45BrN4O6. MW: 709.7. Isolated from Jaspis splendens. Cell permeable, non-fluorescent F-actin probe. Potent inducer of actin polymerization and stabilization. Competes with phallotoxins for actin binding. Antifungal and antiparasitic compound. Antiproliferative and anticancer compound. Apoptosis enhancer/inducer. Tool used for autophagy/phagocytosis research.
- SMILESC[C@H]1C[C@@H](C)\C=C(C)\C[C@H](C)C(=O)N[C@@H](C)C(=O)N(C)[C@H](CC2=C(Br)NC3=C2C=CC=C3)C(=O)N[C@H](CC(=O)O1)C1=CC=C(O)C=C1
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis sp: P. Crews, et al.; Tetrahedron Lett. 27, 2797 (1986)
- Jaspamide, a modified peptide from a Jaspis sponge, with insecticidal and antifungal activity: T.M. Zabriskie, et al.; JACS 108, 3123 (1986)
- Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin: M.R. Bubb, et al.; J. Biol. Chem. 269, 14869 (1994)
- Jasplakinolide's inhibition of the growth of prostate carcinoma cells in vitro with disruption of the actin cytoskeleton: A.M. Senderowicz, et al.; J. Natl. Cancer Inst. 87, 46 (1995)
- Growth modulation and differentiation of acute myeloid leukemia cells by jaspamide: I. Fabian, et al.; Exp. Hematol. 23, 583 (1995)
- Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation: S.C. Posey & B.E. Bierer; J. Biol. Chem. 274, 4259 (1999)
- Alteration of actin organization by jaspamide inhibits ruffling, but not phagocytosis or oxidative burst, in HL-60 cells and human monocytes: I. Fabian, et al.; Blood 93, 3994 (1999)
- Jasplakinolide. An actin-specific reagent that promotes actin polymerization: A. Holzinger; Methods Mol. Biol. 161, 109 (2001) (Review)
- Effect of jasplakinolide on the growth, invasion, and actin cytoskeleton of Plasmodium falciparum: Y. Mizuno, et al.; Parasitol. Res. 88, 844 (2002)
- Induction of apoptosis and CD10/neutral endopeptidase expression by jaspamide in HL-60 line cells: D.P. Cioca & K. Kitano; Cell. Mol. Life Sci. 59, 1377 (2002)
