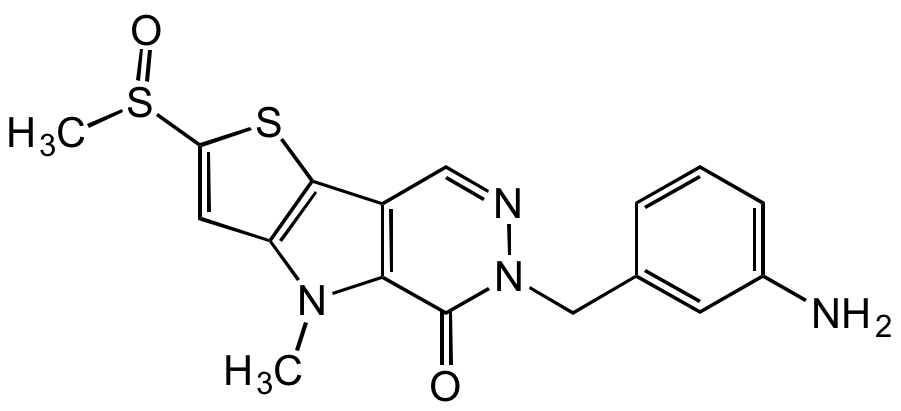
Chemical Structure
TEPP46 [ML-265] [1221186-53-3]

AG-CR1-3687
Overview
- SupplierAdipoGen Life Sciences
- Product NameTEPP46 [ML-265] [1221186-53-3]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationNon-hazardous
- Molecular FormulaC17H16N4O2S2
- Molecular Weight372.5
- Scientific DescriptionChemical. CAS: 1221186-53-3. Formula: C17H16N4O2S2. MW: 372.5. . Potent and selective cell-permeable pyruvate kinase M2 (PKM2) activator with little or no activity versus PKM1, PKL and PKR. Useful agent for immunometabolism research. Anticancer (reducing tumor sizes in mice) and anti-inflammatory agent. Shown to bind the enzyme PKM2 to promote formation of stable, enzymatically-active tetramers, thereby promoting the last rate-limiting step of glycolysis, converting PEP to pyruvate. In addition, it prevents PKM2 translocation to the nucleus resulting in reduced glycolysis and HIF-1alpha activation, causing diminished Warburg effect and IL-1beta production. - Potent and selective cell permeable pyruvate kinase M2 (PKM2) activator with little or no activity versus PKM1, PKL and PKR. Useful agent for immunometabolism research. Anticancer (reducing tumor sizes in mice) and anti-inflammatory agent. Shown to bind the enzyme PKM2 to promote formation of stable, enzymatically-active tetramers, thereby promoting the last rate-limiting step of glycolysis, converting PEP to pyruvate. In addition, it prevents PKM2 translocation to the nucleus resulting in reduced glycolysis and HIF-1alpha activation, causing diminished Warburg effect and IL-1beta production.
- SMILESCS(C1=CC(N2C)=C(C3=C2C(N(N=C3)CC4=CC(N)=CC=C4)=O)S1)=O
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- J.K. Jiang et al. Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase : J.K. Jiang, et al.; Bioorg. Med. Chem. Lett. 20, 3387 (2010)
- ML265: A potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model: M.J. Walsh, et al.; Probe Rep. NIH Mol. Libr. Prog. (2012)
- Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis: D. Anastasiou, et al.; Nat. Chem. Biol. 8, 839 (2012)
- Pyruvate Kinase M2 regulates HIF-1alpha activity and IL-1beta induction and is a critical determinant of the Warburg effect in LPS-activated macrophages: E.M. Palsson-McDermott, et al.; Cell Metab. 21, 65 (2015)
- A guide to immunometabolism for immunologists: L.A. O'Neill, et al.; Nat. Rev. Immunol. 16, 553 (2016)
- Pyruvate Kinase M2: A Potential Target for Regulating Inflammation: J.C. Alves-Filho & E.M. Palsson-McDermott; Front. Immunol. 7, 145 (2016) (Review)
- Similarities and Distinctions of Cancer and Immune Metabolism in Inflammation and Tumors: G. Andrejeva & J.C. Rathmell; Cell Metab. 26, 49 (2017)
- Targeting metabolism as a novel therapeutic approach to autoimmunity, inflammation and transplantation: I.A. Bettencourt & J.D. Powell; J. Immunol. 198, 999 (2017)
- Caloric Restriction Mimetic 2-Deoxyglucose Alleviated Inflammatory Lung Injury via Suppressing Nuclear Pyruvate Kinase M2-Signal Transducer and Activator of Transcription 3 Pathway : K. Hu, et al.; Front. Immunol. 9, 426 (2018)
