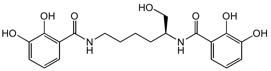
Chemical Structure
Myxochelin A [120243-02-9]

AG-CN2-0470
CAS Number120243-02-9
Product group Chemicals
Estimated Purity>99% (NMR)
Molecular Weight404.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameMyxochelin A [120243-02-9]
- Delivery Days Customer10
- CAS Number120243-02-9
- CertificationResearch Use Only
- Estimated Purity>99% (NMR)
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC20H24N2O7
- Molecular Weight404.4
- Scientific DescriptionChemical. CAS: 120243-02-9. Formula: C20H24N2O7. MW: 404.4. Synthetic. Originally isolated from Pyxidicoccus fallax HKI 727. Potent inhibitor of human 5-lipoxygenase (5-LO). This enzyme catalyzes two initial steps in the conversion of arachidonic acid into leukotrienes, well known mediators of inflammatory and allergic reactions. Iron-chelating compound. Anticancer antibiotic. Shown to inhibit tumor cell invasion in vitro. Antibacterial compound. Antioxidant with free radical scavenging activities. - Potent inhibitor of human 5-lipoxygenase (5-LO). This enzyme catalyzes two initial steps in the conversion of arachidonic acid into leukotrienes, well known mediators of inflammatory and allergic reactions. Iron-chelating compound. Anticancer antibiotic. Shown to inhibit tumor cell invasion in vitro. Antibacterial compound. Antioxidant with free radical scavenging activities.
- SMILESOC[C@H](CCCCNC(=O)C1=C(O)C(O)=CC=C1)NC(=O)C1=CC=CC(O)=C1O
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Myxochelin A, a new iron-chelating compound from Angiococcus disciformis (Myxobacterales). Production, isolation, physico-chemical and biological properties: B. Kunze, et al.; J. Antibiot. 42, 14 (1989)
- Myxochelin A, a cytotoxic antibiotic from the myxobacterium Angiococcus disciformis: J.W. Ahn, et al.; OPEM 2, 64 (2001)
- Antioxidative and free radical scavenging activities of Myxochelin A isolated from Angiococcus sp.(Myxobacteria): H.-H. Lee, et al.; Food Sci. Biotech. 11, 184 (2002)
- Absolute configuration and antitumor activity of myxochelin A produced by Nonomuraea pusilla TP-A0861: S. Miyanaga, et al.; J. Antibiot. 59, 698 (2006)
- Synthesis and evaluation of myxochelin analogues as antimetastatic agents: S. Miyanaga, et al.; Bioorg. Med. Chem. 17, 2724 (2009)
- Myxochelins target human 5-lipoxygenase: S. Schieferdecker, et al.; J. Nat. Prod. 78, 335 (2015)
- Harnessing enzymatic promiscuity in Myxochelin biosynthesis for the production of 5-Lipoxygenase inhibitors: J. Korp, et al.; Chembiochem. 16, 2445 (2015)
