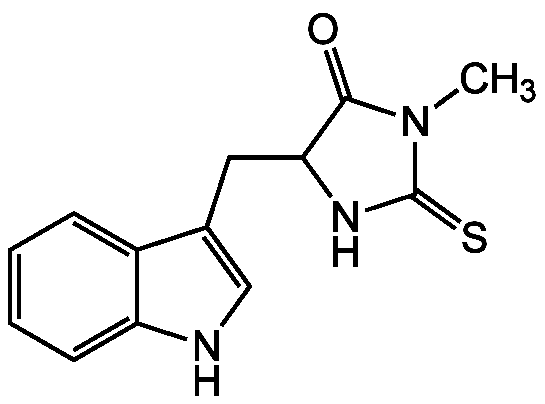
Chemical Structure
Necrostatin-1 [4311-88-0]

AG-CR1-2900
CAS Number4311-88-0
Product group Chemicals
Estimated Purity>98%
Molecular Weight259.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameNecrostatin-1 [4311-88-0]
- Delivery Days Customer10
- CAS Number4311-88-0
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous
- Molecular FormulaC13H13N3OS
- Molecular Weight259.3
- Scientific DescriptionChemical. CAS: 4311-88-0. Formula: C13H13N3OS. MW: 259.3. Competitive indoleamine 2,3-dioxygenase (IDO) inhibitor. Cell permeable, potent and selective necroptosis (a non-apoptotic form of programmed cell death) inhibitor. Showed neuroprotection in a murine model of ischemic brain injury in vivo. Selective and ATP-competitive receptor-interacting protein kinase 1 (RIPK1) inhibitor. Used in inflammatory and degenerative disease models to target RIPK1. Suppresses autophagy and apoptosis in murine brain injury models. Cardioprotective and neuroprotective. - Competitive indoleamine 2,3-dioxygenase (IDO) inhibitor [1, 4, 9, 10]. Cell permeable, potent and selective necroptosis (a non-apoptotic form of programmed cell death) inhibitor [2, 5]. Showed neuroprotection in a murine model of ischemic brain injury in vivo [2, 3]. Selective and ATP-competitive receptor-interacting protein kinase 1 (RIPK1) inhibitor [6, 7]. Used in inflammatory and degenerative disease models to target RIPK1 [7, 9, 10]. Suppresses autophagy and apoptosis in murine brain injury models [8, 9]. Cardioprotective and neuroprotective [11-14].
- SMILESCN1C(=S)NC(CC2=CNC3=CC=CC=C23)C1=O
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy: A.J. Muller, et al.; Nat. Med. 11, 312 (2005)
- Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury: A. Degterev, et al.; Nat. Chem. Biol. 1, 112 (2005)
- Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells: X. Xu, et al.; J. Neurochem. 103, 2004 (2007)
- Clinical aspects of indoleamine 2,3-dioxygenase (IDO)-initiated tryptophan metabolism: IDO is a target of drug discovery for various diseasea: O. Takikawa; Int. Congr. Ser. 1304, 290 (2007)
- Chemical genetic approaches to probing cell death: B.R. Stockwell & N.M. Gangadhar; Curr. Opin. Chem. Biol. 11, 83 (2007)
- Identification of RIP1 kinase as a specific cellular target of necrostatins: A. Degterev, et al.; Nat. Chem. Biol. 4, 313 (2008)
- RIP1-dependent and independent effects of necrostatin-1 in necrosis and T cell activation: Y. Cho, et al.; PLoS One 6, e23209 (2011)
- Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic brain injury model: Y.Q. Wang, et al.; Neurochem. Res. 37, 1849 (2012)
- Necrostatin-1 blocks both RIPK1 and IDO: consequences for the study of cell death in experimental disease models: P. Vandenabeele, et al.; Cell Death Differ. 20, 185 (2013)
- Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase: A. Degterev, et al.; Cell Death Differ 20, 366 (2013)
