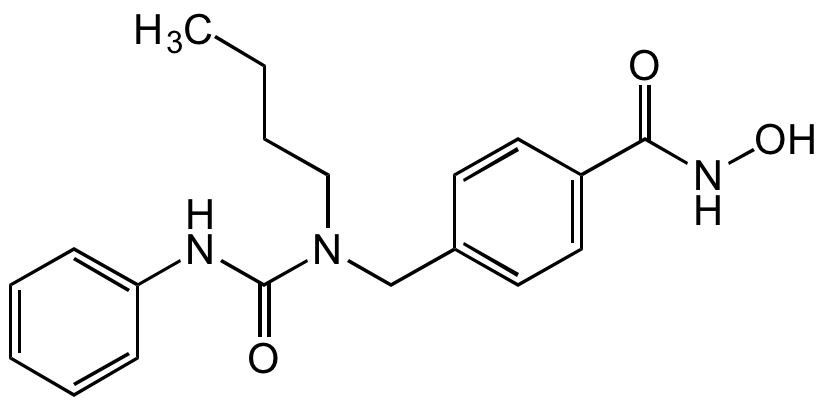
Chemical Structure
Nexturastat A [1403783-31-2]

AG-CR1-3901
CAS Number1403783-31-2
Product group Chemicals
Estimated Purity>98%
Molecular Weight341.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameNexturastat A [1403783-31-2]
- Delivery Days Customer10
- CAS Number1403783-31-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous
- Molecular FormulaC19H23N3O3
- Molecular Weight341.4
- Scientific DescriptionCell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =5.02nM). Displays high selectivity over all other HDACs (IC50=3-10microM). Suppresses cell proliferation and promotes apoptosis in B16 cells (GI50=14.3microM) and human lymphoma cells HuT-78. Dose-dependently induces hyperacetylation of alpha-tubulin in B16 murine melanoma cells without elevating histone H3 acetylation. Induces cell cycle arrest in G1. HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration. - Chemical. CAS: 1403783-31-2. Formula: C19H23N3O3. MW: 341.4. Cell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =5.02nM). Displays high selectivity over all other HDACs (IC50=3-10microM). Suppresses cell proliferation and promotes apoptosis in B16 cells (GI50=14.3microM) and human lymphoma cells HuT-78. Dose-dependently induces hyperacetylation of alpha-tubulin in B16 murine melanoma cells without elevating histone H3 acetylation. Induces cell cycle arrest in G1. HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration.
- SMILESCCCCN(CC1=CC=C(C=C1)C(=O)NO)C(=O)NC1=CC=CC=C1
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth: J.A. Bergman, et al.; J. Med. Chem. 55, 9891 (2012)
- Development and therapeutic implications of selective histone deacetylase 6 inhibitors: J.H. Kalin & J.A Bergman; J. Med. Chem. 56, 6297 (2013)
- Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: Enhanced antitumor immunity and impaired cell proliferation: K.V. Woan, et al.; Mol. Oncol. 9, 1447 (2015)
- Trend of histone deacetylase inhibitors in cancer therapy: Isoform selectivity or multitargeted strategy: L. Zhang, et al.; Med. Res. Rev. 35, 63 (2015) (Review)
