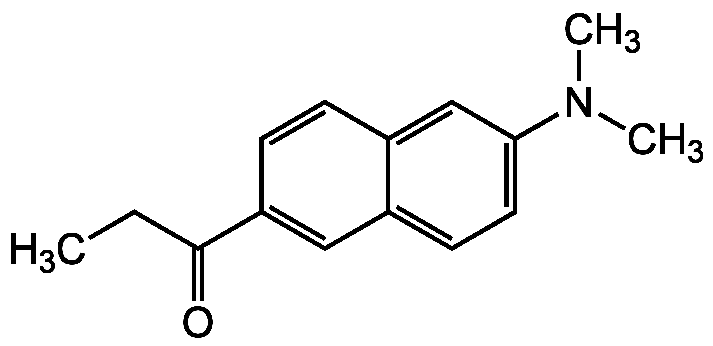
Chemical Structure
N,N-Dimethyl-6-propionyl-2-naphthylamine [70504-01-7]

CDX-D0077
CAS Number70504-01-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight227.3
Overview
- SupplierChemodex
- Product NameN,N-Dimethyl-6-propionyl-2-naphthylamine [70504-01-7]
- Delivery Days Customer10
- CAS Number70504-01-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous
- Molecular FormulaC15H17NO
- Molecular Weight227.3
- Scientific DescriptionChemical. CAS: 70504-01-7. Formula: C15H17NO. MW: 227.3. Synthetic. N,N-dimethyl-6-propionyl-2-naphthylamine (prodan) has both an electron-donor and an electron-acceptor substituent, resulting in a large excited-state dipole moment and extensive solvent polarity-dependent fluorescence shifts. When prodan is incorporated into membranes, their fluorescence spectra are sensitive to the physical state of the surrounding phospholipids. In membranes, prodan appears to localize at the surface, although Fourier transform infrared measurements indicate some degree of penetration into the lipid interior. Excited-state relaxation of prodan is sensitive to the nature of the linkage between phospholipid hydrocarbon tails and the glycerol backbone. Tubulin and its hydrophobic surfaces have been probed with the enviroment-sensitive probes prodan. Prodan is also used for the generation of peroxy-oxalate chemiluminescence with H2O2. - N,N-dimethyl-6-propionyl-2-naphthylamine (prodan) has both an electron-donor and an electron-acceptor substituent, resulting in a large excited-state dipole moment and extensive solvent polarity-dependent fluorescence shifts. When prodan is incorporated into membranes, their fluorescence spectra are sensitive to the physical state of the surrounding phospholipids. In membranes, prodan appears to localize at the surface, although Fourier transform infrared measurements indicate some degree of penetration into the lipid interior. Excited-state relaxation of prodan is sensitive to the nature of the linkage between phospholipid hydrocarbon tails and the glycerol backbone. Tubulin and its hydrophobic surfaces have been probed with the enviroment-sensitive probes prodan. Prodan is also used for the generation of peroxy-oxalate chemiluminescence with H2O2.
- SMILESCCC(=O)C1=CC2=CC=C(C=C2C=C1)N(C)C
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
