Chemical Structure
Ophiobolin A [4611-05-6]

AG-CN2-0431
CAS Number4611-05-6
Product group Chemicals
Estimated Purity>95% (HPLC)
Molecular Weight400.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameOphiobolin A [4611-05-6]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number4611-05-6
- CertificationResearch Use Only
- Estimated Purity>95% (HPLC)
- Hazard InformationDanger,Excepted quantity
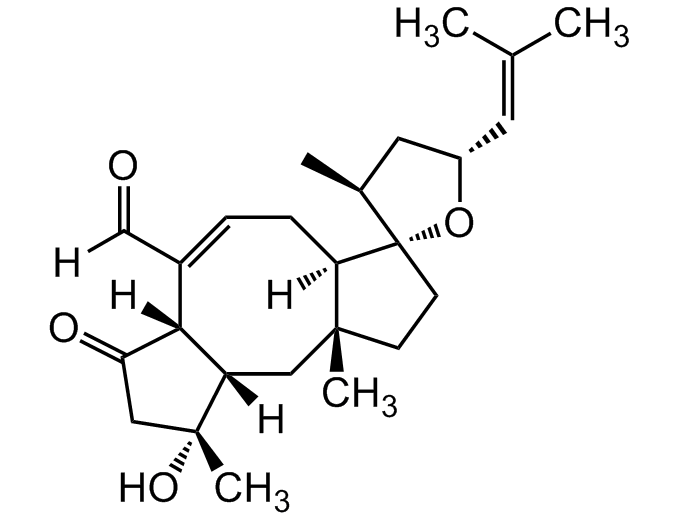
- Molecular FormulaC25H36O4
- Molecular Weight400.6
- Scientific DescriptionCell permeable, irreversible calmodulin antagonist. Acts by covalently binding to a lysine-rich inhibitory site. Inhibits by blocking the activation of the Ca2+/calmodulin-dependent phosphodiesterase [1-3, 5, 6, 9]. Herbicidal mycotoxin [7]. Phytotoxic, antifungal, antibacterial and nematocidal compound [4, 6, 10]. Anticancer compound. Shown to induce paraptosis-like cell death [11, 12]. Shown to inhibit P-glycoprotein-mediated transport [8]. Disruptor of intracellular sulfhydryl proteostasis, leading to ER stress and dilation. - Chemical. CAS: 4611-05-6. Formula: C25H36O4. MW: 400.6. Isolated from Bipolaris leersia. Cell permeable, irreversible calmodulin antagonist. Acts by covalently binding to a lysine-rich inhibitory site. Inhibits by blocking the activation of the Ca2+/calmodulin-dependent phosphodiesterase. Herbicidal mycotoxin. Phytotoxic, antifungal, antibacterial and nematocidal compound. Anticancer compound. Shown to induce paraptosis-like cell death. Shown to inhibit P-glycoprotein-mediated transport.
- SMILES[H]C(=O)C1=C[C@]2([H])[C@](C)(CC[C@@]22O[C@H](C[C@@H]2C)C=C(C)C)C[C@@]2([H])[C@]1([H])C(=O)C[C@@]2(C)O
- Storage Instruction2°C to 8°C,-20°C
- UN NumberUN 3462
- UNSPSC12352200
References
- Ophiobolin A. A natural product inhibitor of calmodulin: P.C. Leung, et al.; J. Biol. Chem. 259, 2742 (1984)
- Role of Calmodulin Inhibition in the Mode of Action of Ophiobolin A: P.C. Leung, et al.; Plant Physiol. 77, 303 (1985)
- Characterization of the interaction of ophiobolin A and calmodulin: P.C. Leung, et al.; Int. J. Biochem. 20, 1351 (1988)
- Microbial metabolites of ophiobolin A and antimicrobial evaluation of ophiobolins: E. Li, et al.; J. Nat. Prod. 58, 74 (1995)
- Identification of the binding and inhibition sites in the calmodulin molecule for ophiobolin A by site-directed mutagenesis: T. Kong Au & P. Chow Leung; Plant Physiol. 118, 965 (1998)
- The biology of ophiobolins: T.K. Au, et al.; Life Sci. 67, 733 (2000) (Review)
- Herbicidal potential of ophiobolins produced by Drechslera gigantea: A. Evidente, et al.; J. Agric. Food Chem. 54, 1779 (2006)
- Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products: N. Yoshida, et al.; Food Chem. Toxicol. 44, 2033 (2006)
- Calcium depletion and calmodulin inhibition affect the import of nuclear-encoded proteins into plant mitochondria: S. Kuhn, et al.; Plant J. 58, 694 (2009)
- Effect of the sesterterpene-type metabolites, ophiobolins A and B, on zygomycetes fungi: K. Krizsan, et al.; FEMS Microbiol. Lett. 313, 135 (2010)
