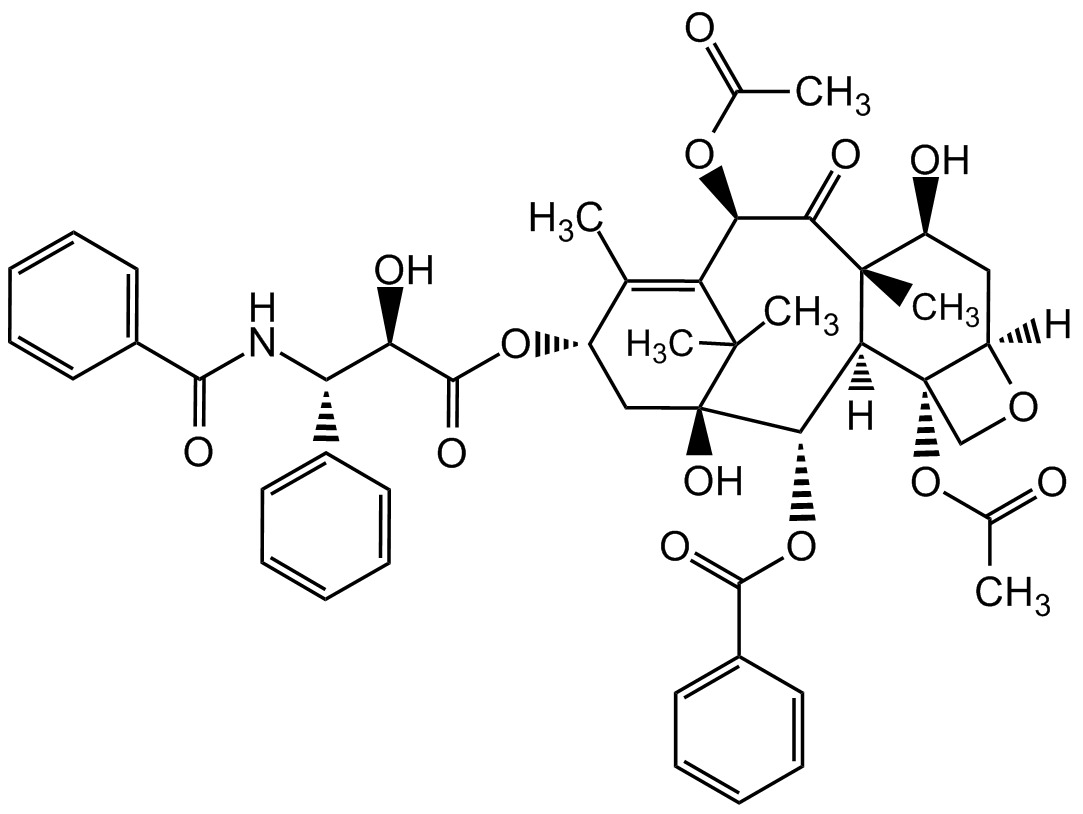
Chemical Structure
Paclitaxel [33069-62-4]

AG-CN2-0045
CAS Number33069-62-4
Product group Chemicals
Estimated Purity>99%
Molecular Weight853.9
Overview
- SupplierAdipoGen Life Sciences
- Product NamePaclitaxel [33069-62-4]
- Delivery Days Customer10
- CAS Number33069-62-4
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationDanger,Non-hazardous
- Molecular FormulaC47H51NO14
- Molecular Weight853.9
- Scientific DescriptionAnticancer compound [1, 11, 14]. Chemotherapeutic used in patients with cancer and advanced forms of Kaposis sarcoma [11, 12]. Microtubule assembly stabilizer. Reversibly binds to polymerized tubulin [2, 3, 6, 13, 14]. Anti-mitotic. Mitotic spindle assembly, chromosome segregation and cell division inhibitor. Induces cell cycle arrest at the G2/M phase [4, 5, 10, 13]. Apoptosis inducer through aberrant activation of cyclin-dependent kinases (CKDs) and the c-Jun N-terminal kinase/stress activated protein kinase (JNK/SAPK) [7-9, 10]. Immunosuppressor. Immunostimulant [15]. Antiproliferative agent for the prevention of restenosis [16]. TRAIL sensitizer [17] - Chemical. CAS: 33069-62-4. Formula: C47H51NO14. MW: 853.9. Isolated from the bark of the pacific yew tree (Taxus brevifolia). Anticancer compound. Chemotherapeutic used in patients with cancer and advanced forms of Kaposis sarcoma. Microtubule assembly stabilizer. Reversibly binds to polymerized tubulin. Anti-mitotic. Mitotic spindle assembly, chromosome segregation and cell division inhibitor. Induces cell cycle arrest at the G2/M phase. Apoptosis inducer through aberrant activation of cyclin-dependent kinases (CKDs) and the c-Jun N-terminal kinase/stress activated protein kinase (JNK/SAPK). Immunosuppressor. Immunostimulant. Antiproliferative agent for the prevention of restenosis. TRAIL sensitizer
- SMILES[H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)C5=CC=CC=C5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)C1=CC=CC=C1)C1=CC=CC=C1
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Cytotoxic studies of paclitaxel (Taxol®) in human tumour cell lines: J.E. Liebmann, et al.; Br. J. Cancer 68, 1104 (1993)
- Taxol (paclitaxel): mechanisms of action: S.B. Horwitz; Ann. Oncol. 5, S3 (1994) (Review)
- Taxol (paclitaxel): a novel anti-microtubule agent with remarkable anti-neoplastic activity: R. Foa, et al.; Int. J. Clin. Lab. Res. 24, 6 (1994) (Review)
- Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway: C.M. Woods, et al.; Mol. Med. 1, 506 (1995)
- Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle: A. Jordan, et al.; Med. Res. Rev. 18, 259 (1998)
- How Taxol stabilises microtubule structure: L.A. Amos & J. Löwe; Chem. Biol. 6, R65 (1999) (Review)
- Mechanisms of Taxol-induced cell death are concentration dependent: K. Torres & S.B. Horwitz; Cancer Res. 58, 3620 (1998)
- Molecular effects of paclitaxel: myths and reality (a critical review): M.V. Blagosklonny & T. Fojo; Int. J. Cancer 83, 151 (1999)
- Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK) dependent and -independent pathways in ovarian cancer cells: T.H. Wang, et al.; J. Biol. Chem. 274, 8208 (1999)
- Paclitaxel-induced cell death: where the cell cycle and apoptosis come together: T.H. Wang, et al.; Cancer 88, 2619 (2000)
