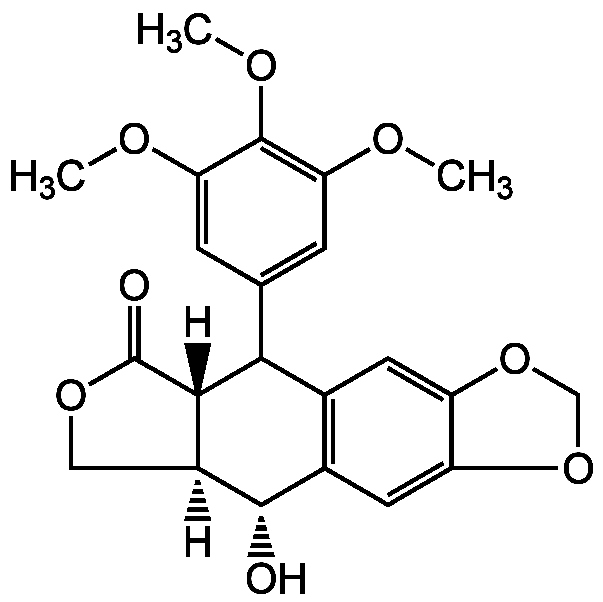
Chemical Structure
Podophyllotoxin [518-28-5]

AG-CN2-0049
CAS Number518-28-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight414.4
Overview
- SupplierAdipoGen Life Sciences
- Product NamePodophyllotoxin [518-28-5]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number518-28-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC22H22O8
- Molecular Weight414.4
- Scientific DescriptionChemical. CAS: 518-28-5. Formula: C22H22O8. MW: 414.4. Isolated from Podophyllum emodi rhizomes. Potent microtubule assembly inhibitor. Anticancer compound. Cell death inducer. DNA topoisomerase II inhibitor. Cell cycle inhibitor at the metaphase. Antiviral and antihelminthic. - Potent microtubule assembly inhibitor [1-3]. Anticancer compound [1-5, 7]. Cell death inducer [1-5, 7]. DNA topoisomerase II inhibitor. Cell cycle inhibitor at the metaphase [2, 6, 8]. Antiviral [3, 4, 9] and antihelminthic [1].
- SMILES[H][C@]12COC(=O)[C@]1([H])C(C1=CC(OC)=C(OC)C(OC)=C1)C1=C(C=C3OCOC3=C1)[C@@H]2O
- Storage Instruction2°C to 8°C,-20°C
- UN NumberUN 2811
- UNSPSC12352200
References
- VP16-213 and podophyllotoxin. A study on the relationship between chemical structure and biological activity: J.D. Loike; Cancer Chemother. Pharmacol. 7, 103 (1982)
- Antitumor agents. I. DNA topoisomerase II inhibitory activity and the structural relationship of podophyllotoxin derivatives as antitumor agents: T. Terada, et al.; Chem. Pharmacol. Bull. (Tokyo) 40, 2720 (1992)
- Antineoplastic and antiviral activities of podophyllotoxin related lignans: M. Gordaliza, et al.; Arch. Pharmacol. 327, 175 (1994)
- Podophyllotoxin: C. Canel, et al.; Phytochemistry 54, 115 (2000) (Review)
- Antitumor properties of podophyllotoxin and related compounds: M. Gordaliza, et al.; Curr. Pharm. Des. 6, 1811 (2000) (Review)
- Drugs that inhibit tubulin polymerization: the particular case of podophyllotoxin and analogues: S. Desbene & S. Giorgi-Renault; Curr. Med. Chem. Anticancer Agents 2, 71 (2002) (Review)
- Podophyllotoxin derivatives: current synthetic approaches for new anticancer agents: Y. You; Curr. Pharm. Des. 11, 1695 (2005) (Review)
- Camptothecin and podophyllotoxin derivatives: inhibitors of topoisomerase I and II - mechanisms of action, pharmacokinetics and toxicity profile: J.T. Hartmann & H.P. Lipp; Drug Saf. 29, 209 (2006) (Review)
- An evidence-based review of medical and surgical treatments of genital warts: N. Scheinfeld & D.S. Lehman; Dermatol. Online J. 12, 5 (2006)
