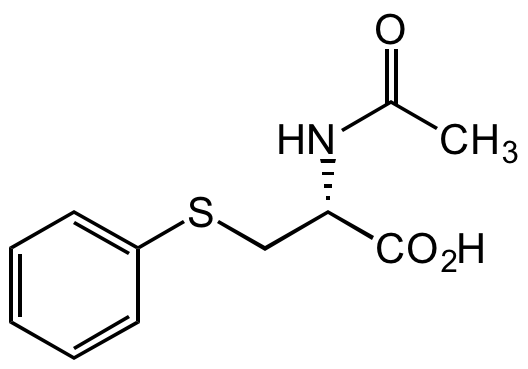
Chemical Structure
S-Phenylmercapturic acid [4775-80-8]

CDX-P0091
CAS Number4775-80-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight239.29
Overview
- SupplierChemodex
- Product NameS-Phenylmercapturic acid [4775-80-8]
- Delivery Days Customer10
- CAS Number4775-80-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous
- Molecular FormulaC11H13NO3S
- Molecular Weight239.29
- Scientific DescriptionChemical. CAS: 4775-80-8. Formula: C11H13NO3S. MW: 239.29. Synthetic. S-Phenylmercapturic acid (S-PMA) is an important metabolite of benzene used as a biomarker for benzene exposure in humans. In industrial workers, urinary concentrations of up to 543 microg/g creatinine have been detected. In a study of benzene exposure through tobacco smoke inhalation in humans, smokers had a urinary concentration of 9.1 microg S-PMA/g creatinine compared with 4.8 microg S-PMA/g creatinine in non-smokers. S-PMA can be sensitively and reliably determined using capillary gas chromatographic/mass spectrometric method. Due to its sensitivity the method is suitable not only for determining concentrations relevant to occupational medicine, but also those which occur in the ecological range. Compound can be used as analytical reference material. - S-Phenylmercapturic acid (S-PMA) is an important metabolite of benzene used as a biomarker for benzene exposure in humans. In industrial workers, urinary concentrations of up to 543 microg/g creatinine have been detected. In a study of benzene exposure through tobacco smoke inhalation in humans, smokers had a urinary concentration of 9.1 microg S-PMA/g creatinine compared with 4.8 microg S-PMA/g creatinine in non-smokers. S-PMA can be sensitively and reliably determined using capillary gas chromatographic/mass spectrometric method. Due to its sensitivity the method is suitable not only for determining concentrations relevant to occupational medicine, but also those which occur in the ecological range. Compound can be used as analytical reference material.
- SMILESCC(N[C@H](C(O)=O)CSC1=CC=CC=C1)=O
- Storage Instruction-20°C
- UNSPSC12352200
