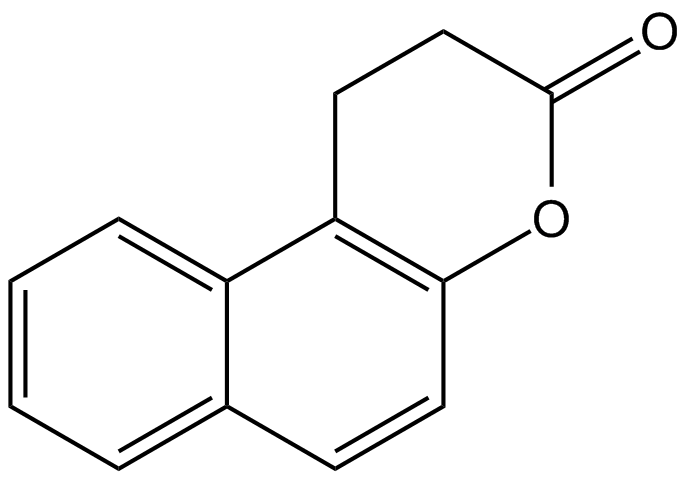
Chemical Structure
Splitomicin [5690-03-9]

AG-CR1-0088
Overview
- SupplierAdipoGen Life Sciences
- Product NameSplitomicin [5690-03-9]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>98% (NMR)
- Hazard InformationNon-hazardous
- Molecular FormulaC13H10O2
- Molecular Weight198.2
- Scientific DescriptionChemical. CAS: 5690-03-9. Formula: C13H10O2. MW: 198.2. Potent cell permeable and selective inhibitor of yeast NAD+-dependent histone deacetylase (HDAC) Sir2p. Displays higher activity in vivo than in vitro. Sensitizes mammalian cells to a variety of DNA-damaging agents by abrogating Sir2p activity on p53. Acts by either altering or blocking access to the acetylated histone binding pocket. Shown to have diverse effects also in mammalian cells. - Potent cell permeable and selective inhibitor of yeast NAD+-dependent histone deacetylase (HDAC) Sir2p [1-4]. Displays higher activity in vivo than in vitro [1-4]. Sensitizes mammalian cells to a variety of DNA-damaging agents by abrogating Sir2p activity on p53. Acts by either altering or blocking access to the acetylated histone binding pocket [5]. Shown to have diverse effects also in mammalian cells [6-10].
- SMILESO=C1CCC2=C(O1)C=CC1=CC=CC=C21
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Identification of a small molecule inhibitor of Sir2p: A. Bedalov, et al.; PNAS 98, 15113 (2001)
- Identification of selective inhibitors of NAD+-dependent deacetylases using phenotypic screens in yeast: M. Hirao, et al.; J. Biol. Chem. 278, 52773 (2003)
- Inhibitors of Sir2: evaluation of splitomicin analogues: J. Posakony, et al.; J. Med. Chem. 47, 2635 (2004)
- The Sir 2 family of protein deacetylases: J.M. Denu; Curr. Opin. Chem. Biol. 9, 431 (2005) (Review)
- Histone deacetylase inhibitor-mediated radiosensitization of human cancer cells: class differences and the potential influence of p53: I.A. Kim, et al.; Clin. Cancer Res. 12, 940 (2006)
- SIRT1 inhibition alleviates gene silencing in Fragile X mental retardation syndrome: R. Biacsi, et al.; PLoS Genet. 4, e1000017 (2008)
- Splitomicin suppresses human platelet aggregation via inhibition of cyclic AMP phosphodiesterase and intracellular Ca++ release: F.C. Liu, et al.; Thromb. Res. 124, 199 (2009)
- Reciprocal roles of SIRT1 and SKIP in the regulation of RAR activity: implication in the retinoic acid-induced neuronal differentiation of P19 cells: M.R. Kang, et al.; Nucleic Acids Res. 38, 822 (2010)
- Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells: A. Breitenstein, et al.; Cardiovasc. Res. 89, 464 (2011)
- Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival: U.H. Beier, et al.; Mol. Cell Biol. 31, 1022 (2011)
