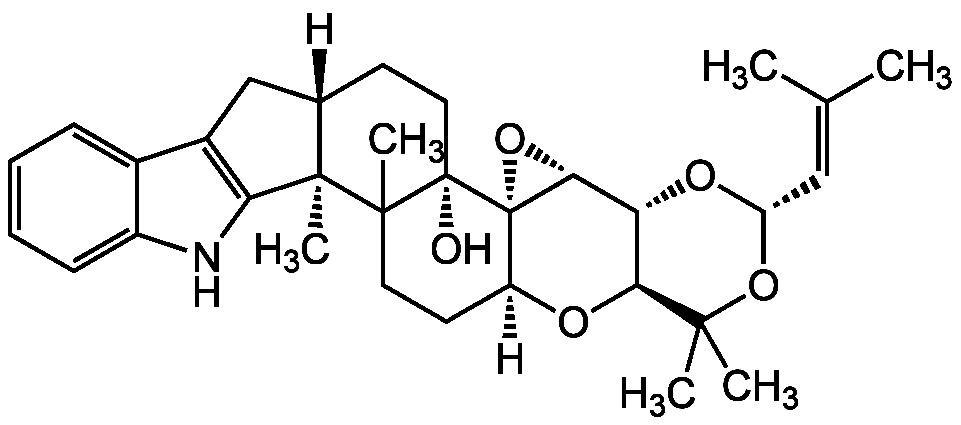
Chemical Structure
Terpendole C [156967-65-6]

AG-CN2-0125
CAS Number156967-65-6
Product group Chemicals
Estimated Purity>95% (HPLC)
Molecular Weight519.7
Overview
- SupplierAdipoGen Life Sciences
- Product NameTerpendole C [156967-65-6]
- Delivery Days Customer10
- CAS Number156967-65-6
- CertificationResearch Use Only
- Estimated Purity>95% (HPLC)
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC32H41NO5
- Molecular Weight519.7
- Scientific DescriptionAcyl-CoA:cholesterol acyltransferase (ACAT) isozymes ACAT1 and ACAT2 inhibitor [1-3, 6]. Tremorgenic [4, 5]. Cholesteryl ester (CE) synthesis inhibitor [6, 7]. - Chemical. CAS: 156967-65-6. Formula: C32H41NO5. MW: 519.7. Isolated from Albophoma yamanashiensis. Acyl-CoA:cholesterol acyltransferase (ACAT) isozymes ACAT1 and ACAT2 inhibitor. Tremorgenic. Cholesteryl ester (CE) synthesis inhibitor.
- SMILES[H][C@]12CC3=C(NC4=CC=CC=C34)[C@]1(C)C1(C)CC[C@]3([H])O[C@H]4[C@@H](O[C@@H](OC4(C)C)C=C(C)C)[C@H]4O[C@@]34[C@]1(O)CC2
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Microbial metabolites affecting lipid biosynthesis: S. Omura & H. Tomoda; Pure Appl. Chem. 66, 2267 (1994)
- Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. I. Production, isolation and biological properties: X.H. Huang, et al.; J. Antibiot. (Tokyo) 48, 1 (1995)
- Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. II. Structure elucidation of terpendoles A, B, C and D: X.H. Huang, et al.; J. Antibiot. (Tokyo) 48, 5 (1995)
- Isolation and structure elucidation of lolilline, a possible biosynthetic precursor of the lolitrem family of tremorgenic mycotoxins: S.C. Munday-Finch, et al.; J. Agric. Food Chem. 45, 199 (1997)
- Terpendole M, a novel indole-diterpenoid isolated from Lolium perenne infected with the endophytic fungus Neotyphodium lolii: W.A. Gatenby, et al.; J. Agric. Food Chem. 47, 1092 (1999)
- Selectivity of microbial acyl-CoA:cholesterol acyltransferase inhibitors toward isozymes: T. Ohshiro, et al.; J. Antibiot. (Tokyo) 60, 43 (2007)
- Potential therapeutics for obesity and atherosclerosis: Inhibitors of neutral lipid metabolism from microorganisms: H. Tomoda & S. Omura; Pharmacol. Ther. 115, 375 (2007)
