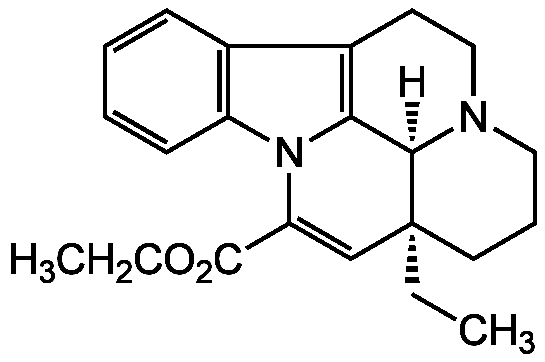
Chemical Structure
Vinpocetine [42971-09-5]

AG-CN2-0454
CAS Number42971-09-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight350.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameVinpocetine [42971-09-5]
- Delivery Days Customer10
- CAS Number42971-09-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC22H26N2O2
- Molecular Weight350.5
- Scientific DescriptionChemical. CAS: 42971-09-5. Formula: C22H26N2O2. MW: 350.5. Semi-synthetic from Voacanga africana. Selective Ca2+-calmodulin dependent cGMP-phosphodiesterase (PDE1) inhibitor. Shows vasorelaxant activity. Neuroprotective agent. Selectively inhibits voltage-sensitive 2+ channels Potent anti-inflammatory agent. Inhibitor of NF-kappaB-dependent inflammatory responses by directly targeting IKK. Shown to inhibit the NLRP3 inflammasome. Antioxidant. Free radical scavenger. Anticancer compound. Anticonvulsant. - Selective Ca2+-calmodulin dependent cGMP-phosphodiesterase (PDE1) inhibitor. Shows vasorelaxant activity. Neuroprotective agent. Selectively inhibits voltage-sensitive Na2+ channels Potent anti-inflammatory agent. Inhibitor of NF-kappaB-dependent inflammatory responses Inhibitor of IkappaB kinase IKK. Shown to inhibit the NLRP3 inflammasome. Antioxidant. Free radical scavenger. Anticancer compound. Anticonvulsant. Attenuates osteoblastic differentiation of vascular smooth muscle cells. Anti-schistosomal agent. Attenuates liver fibrosis
- SMILESCC[C@@]12CCCN3[C@@H]1C4=C(CC3)C5=CC=CC=C5N4C(=C2)C(=O)OCC
- Storage InstructionRT
- UNSPSC12352200
References
- Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta: H.S. Ahn, et al.; Biochem. Pharmacol. 38, 3331 (1989)
- Role of selective cyclic GMP phosphodiesterase inhibition in the myorelaxant actions of M&B 22,948, MY-5445, vinpocetine and 1-methyl-3-isobutyl-8-(methylamino)xanthine: J.E. Souness, et al.; Br. J. Pharmacol. 98, 725 (1989)
- Vinpocetine selectively inhibits neurotransmitter release triggered by sodium channel activation: M. Sitges & V. Nekrassov; Neurochem. Res. 24, 1585 (1999)
- In vitro antioxidant properties of pentoxifylline, piracetam, and vinpocetine: B. Horvath, et al.; Clin. Neuropharmacol. 25, 37 (2002)
- Effects of Vinpocetine on mitochondrial function and neuroprotection in primary cortical neurons: K. Tarnok, et al.; Neurochem. Int. 53, 289 (2008)
- Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine - a PDE1 inhibitor: R. Deshmukh, et al.; Eur. J. Pharmacol. 620, 49 (2009)
- Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism: K.I. Jeon, et al.; PNAS 107, 9795 (2010)
- Vinpocetine as a potent antiinflammatory agent: A.E. Medina, et al.; PNAS 107, 9921 (2010)
- Vinpocetine inhibits breast cancer cells growth in vitro and in vivo: E.W. Huang, et al.; Apoptosis 17, 1120 (2012)
- Vinpocetine attenuates lipid accumulation and atherosclerosis formation: Y. Cai, et al.; BBRC 434, 439 (2013)
