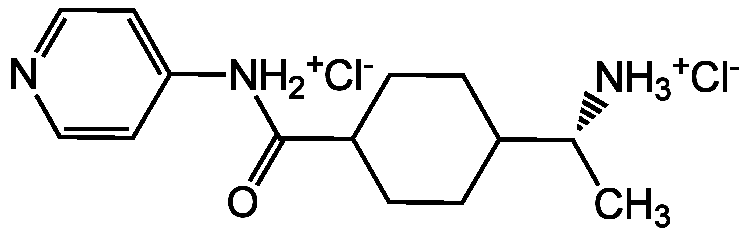
Chemical Structure
Y-27632 . dihydrochloride [129830-38-2, 146986-50-7]

AG-CR1-3564
Estimated Purity>98% (HPLC)Contains approx. 1.4% water.
Product group Chemicals
Molecular Weight247.3 . 72.9
Overview
- SupplierAdipoGen Life Sciences
- Product NameY-27632 . dihydrochloride [129830-38-2, 146986-50-7]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>98% (HPLC)Contains approx. 1.4% water.
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC14H21N3O . 2HCl
- Molecular Weight247.3 . 72.9
- Scientific DescriptionChemical. CAS: 129830-38-2 - 146986-50-7 (free base). Formula: C14H21N3O . 2HCl. MW: 247.3 . 72.9. Synthetic. Potent, cell permeable, selective and ATP-competitive Rho-associated protein kinases inhibitor, including p160ROCK, ROCK-II and PRK2 inhibitor [1,3-5]. Tumor cell invasion and metastasis suppressor [2, 15]. Smooth muscle relaxant [1, 6]. Decreases liver fibrosis by hepatic stellate cell growth inhibition [7, 8]. Antinociceptive [9]. Blocks generation of inflammatory cytokines [10]. Stem cell research modulator [11-14, 16]. Prevents apoptosis and enhances the survival and cloning efficiency of dissociated human embryonic stem (hES) cells without affecting their pluripotency. Increases survival rate of hES cells undergoing cryopreservation [11-14, 16]. - Potent, cell permeable, selective and ATP-competitive Rho-associated protein kinases inhibitor, including p160ROCK, ROCK-II and PRK2 inhibitor [1,3-5]. Tumor cell invasion and metastasis suppressor [2, 15]. Smooth muscle relaxant [1, 6]. Decreases liver fibrosis by hepatic stellate cell growth inhibition [7, 8]. Antinociceptive [9]. Blocks generation of inflammatory cytokines [10]. Stem cell research modulator [11-14, 16]. Prevents apoptosis and enhances the survival and cloning efficiency of dissociated human embryonic stem (hES) cells without affecting their pluripotency. Increases survival rate of hES cells undergoing cryopreservation [11-14, 16].
- SMILESC[C@@H](N)C1CCC(CC1)C(=O)NC1=CC=NC=C1
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension: M. Uehata, et al.; Nature 389, 990 (1997)
- An essential part for Rho-associated kinase in the transcellular invasion of tumor cells: K. Itoh, et al.; Nat. Med. 5, 221 (1999)
- Use and properties of ROCK-specific inhibitor Y-27632: S. Narumiya, et al.; Meth. Enzymol. 325, 273 (2000)
- Specificity and mechanism of action of some commonly used protein kinase inhibitors: S.P. Davies, et al.; Biochem. J. 351, 95 (2000)
- Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases: T. Ishizaki, et al.; Mol. Pharmacol. 57, 976 (2000)
- Y-27632 potentiates relaxant effects of beta 2-adrenoceptor agonists in bovine tracheal smooth muscle: T. Nakahara, et al.; Eur. J. Pharmacol. 389, 103 (2000)
- A p160ROCK-specific inhibitor, Y-27632, attenuates rat hepatic stellate cell growth: H. Iwamoto, et al.; J. Hepatol. 32, 762 (2000)
- Inhibition of intrahepatic metastasis of human hepatocellular carcinoma by Rho-associated protein kinase inhibitor Y-27632: M. Takamura, et al.; Hepatology 33, 577 (2001)
- Rho-kinase inhibitor, Y-27632, has an antinociceptive effect in mice: K. Buyukafsar, et al.; Eur. J. Pharmacol. 541, 49 (2006)
- Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities: C. Doe, et al.; JPET 320, 89 (2007)
