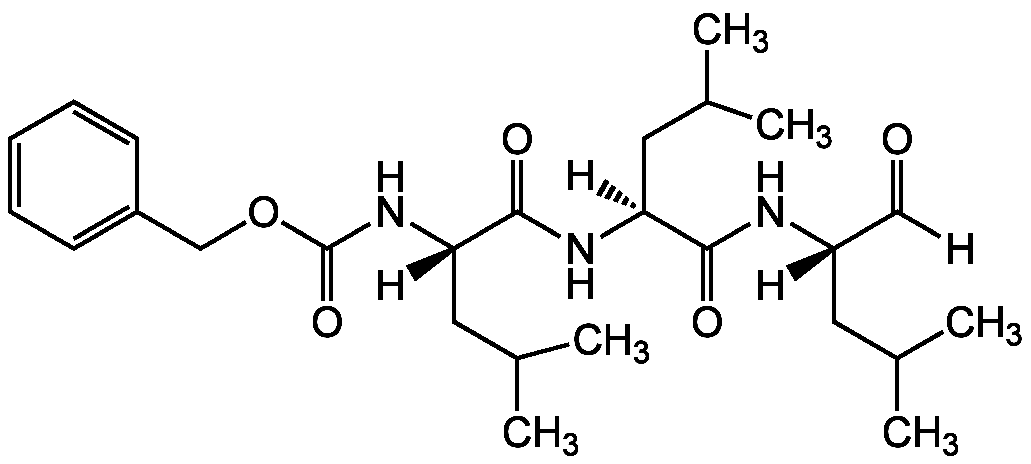
Chemical Structure
Z-Leu-Leu-Leu-CHO [MG-132] [133407-82-6]

AG-CP3-0011
Estimated Purity>97% (HPLC)
Product group Chemicals
Molecular Weight475.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameZ-Leu-Leu-Leu-CHO [MG-132] [133407-82-6]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>97% (HPLC)
- Hazard InformationNon-hazardous
- Molecular FormulaC26H41N3O5
- Molecular Weight475.6
- Scientific DescriptionChemical. CAS: 133407-82-6. Formula: C26H41N3O5. MW: 475.6. Potent, cell permeable and selective, reversible proteasome inhibitor. Blocks degradation of short-lived proteins and induces a heat shock response. NF-kappaB activation inhibitor through IkappaB degradation. Cell permeable, reversible peptide aldehyde inhibitor. Calpain and cathepsin inhibitor. Has anticancer properties by inducing cell cycle arrest and activating apoptosis in various cancer cell lines. Has adjuvant/chemosensitizer potential. Neurite outgrowth stimulator in PC12 cells. Prevents beta-secretase cleavage. Autophagy activator. - Potent, cell permeable and selective, reversible proteasome inhibitor [2, 7]. Blocks degradation of short-lived proteins and induces a heat shock response [2, 6, 7]. NF-kappaB activation inhibitor through IkappaB degradation [3, 8]. Cell permeable, reversible peptide aldehyde inhibitor. Calpain and cathepsin inhibitor [4, 11]. Has anticancer properties by inducing cell cycle arrest and activating apoptosis in various cancer cell lines [8, 9, 12,13,15]. Has adjuvant/chemosensitizer potential [8]. Neurite outgrowth stimulator in PC12 cells [1]. Prevents beta-secretase cleavage [5, 10]. Autophagy activator [12, 14].
- SMILES[H]C(=O)[C@]([H])(CC(C)C)NC(=O)[C@]([H])(CC(C)C)NC(=O)[C@]([H])(CC(C)C)NC(=O)OCC1=CC=CC=C1
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Isolation and characterization of possible target proteins responsible for neurite outgrowth induced by a tripeptide aldehyde in PC12H cells: Y. Saito, et al.; BBRC 184, 419 (1992)
- Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules: K.L. Rock, et al.; Cell 78, 761 (1994)
- The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B: V.J. Palombella, et al.; Cell 78, 773 (1994)
- Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine: S. Tsubuki, et al.; J. Biochem. 119, 572 (1996)
- The carboxyl termini of beta-amyloid peptides 1-40 and 1-42 are generated by distinct gamma-secretase activities: H. Klafki, et al.; J. Biol. Chem. 271, 28655 (1996)
- Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance: K.T. Bush, et al.; J. Biol. Chem. 272, 9086 (1997)
- Proteasome inhibitors: valuable new tools for cell biologists: D.H. Lee & A.L. Goldberg; Trends Cell Biol. 8, 397 (1998)
- Inhibition of NF-kappaB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin: A. Arlt, et al.; Oncogene 20, 859 (2001)
- Potential of the proteasomal inhibitor MG-132 as an anticancer agent, alone and in combination: D. Banerjee & A. Liefshitz; Anticancer Res. 21, 3941 (2001)
- The protease inhibitor, MG132, blocks maturation of the amyloid precursor protein Swedish mutant preventing cleavage by beta-Secretase: M.L. Steinhilb, et al.; J. Biol. Chem. 276, 4476 (2001)
