Chemical Structure
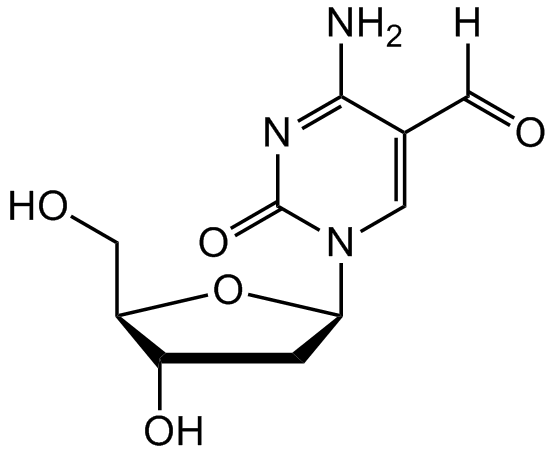
2-Deoxy-5-formyl-cytidine [137017-45-9]

AG-CR1-3531
Overview
- SupplierAdipoGen Life Sciences
- Product Name2-Deoxy-5-formyl-cytidine [137017-45-9]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous
- Molecular FormulaC10H13N3O5
- Molecular Weight255.2
- Scientific DescriptionChemical. CAS: 137017-45-9. Formula: C10H13N3O5. MW: 255.2. Synthetic. Epigenetic base. A minor nucleotide found in DNA and produced by UV or gamma-mediated oxidation of the nucleoside 5-Methyl-2-deoxycytidine. This nucleoside derivative was found in embryonic stem cell DNA. Mutagenic and can induce 5-fC >> T transition mutation as well as the 5-fC >> A and 5-fC >> G transversion mutations during DNA synthesis, particularly in methylated CpG regions. Used in epigenetic research and important for cancer research. - Epigenetic base. A minor nucleotide found in DNA and produced by UV or gamma-mediated oxidation of the nucleoside 5-Methyl-2-deoxycytidine. This nucleoside derivative was found in embryonic stem cell DNA. Mutagenic and can induce 5-fC >> T transition mutation as well as the 5-fC >> A and 5-fC >> G transversion mutations during DNA synthesis, particularly in methylated CpG regions. Used in epigenetic research and important for cancer research.
- SMILESO[C@H]1C[C@H](N2C=C(C([H])=O)C(N)=NC2=O)O[C@@H]1CO
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Formation of 5-formyl-2'-deoxycytidine from 5-methyl-2'-deoxycytidine in duplex DNA by Fenton-type reactions and gamma-irradiation: N. Murata-Kamiya, et al.; Nucleic Acids Res. 27, 4385 (1999)
- Synthesis and properties of oligonucleotides containing 5-formyl-2'-deoxycytidine: in vitro DNA polymerase reactions on DNA templates containing 5-formyl-2'-deoxycytidine: N. Karino, et al.; Nucleic Acids Res. 29, 2456 (2001)
- The discovery of 5-formylcytosine in embryonic stem cell DNA: T. Pfaffeneder, et al.; Angew. Chem. Int. Edit. 50, 7008 (2011)
- A.S. Synthesis of a DNA promoter segment containing all four epigenetic nucleosides: 5-Methyl-, 5-hydroxymethyl-, 5-formyl-, and 5-carboxy-2'-deoxycytidine: Schroeder, et al.; Angew. Chem. Int. Ed. 53, 315 (2014)
- CDA directs metabolism of epigenetic nucleosides revealing a therapeutic window in cancer; M. Zauri, et al.; Nature 524, 114 (2015)
