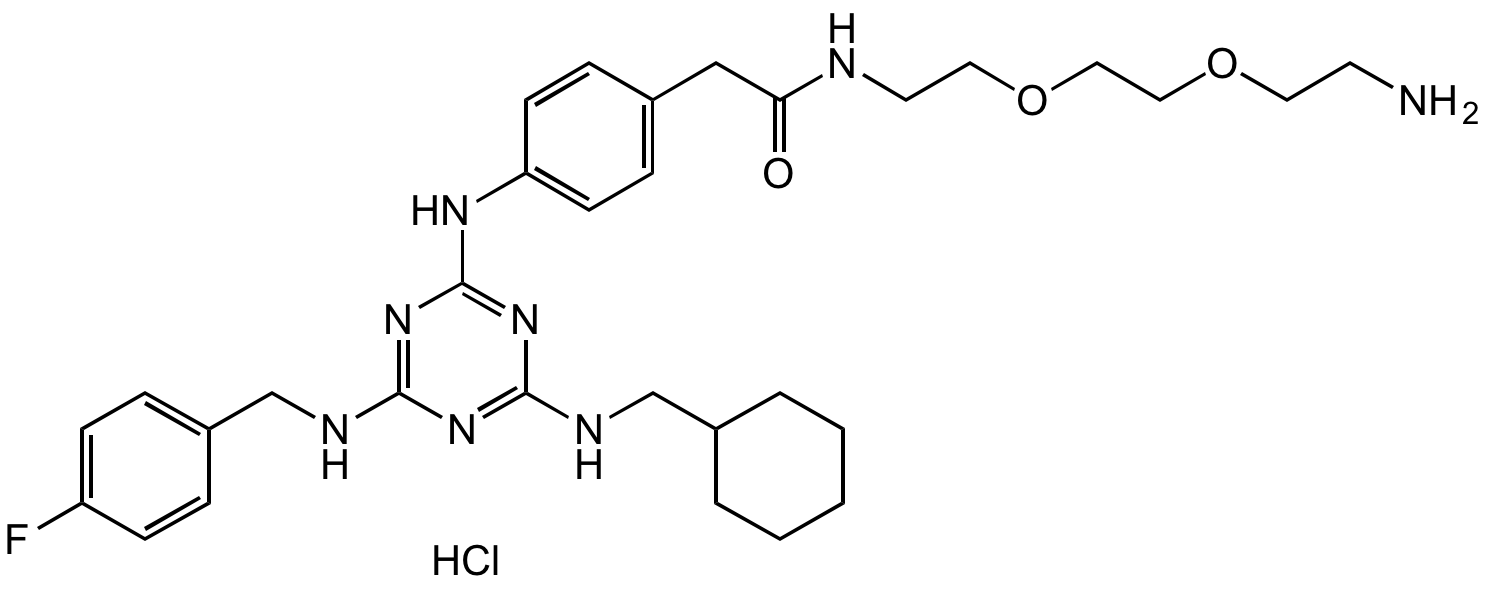
Chemical Structure
AP-III-a4 hydrochloride [1177827-73-4]

AG-CR1-3696
Estimated Purity>95%
Product group Chemicals
Molecular Weight594.7 . 36.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameAP-III-a4 hydrochloride [1177827-73-4]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationNon-hazardous,Warning
- Molecular FormulaC31H43FN8O3 . HCl
- Molecular Weight594.7 . 36.5
- Scientific DescriptionCell permeable first non-substrate analog enolase inhibitor (IC50=0.576microM). A recent study suggested no direct blocking of enolase activity in vitro and a indirect mechanism. Useful agent for immunometabolism research. The glycolysis enzymes enolase is known to have additional, non-glycolytic roles in cellular physiology, which has been termed moonlighting. Anticancer agent. Inhibits cancer cell metastasis in a zebrafish cancer cell xenograft model. Induces cell death under hypoxia and inhibits cancer cell migration and invasion by down-regulation of AKT and Bcl-xL expression. Antidiabetic agent. Reduced hyperglycemia and hyperlipidemia in mice. Reduced blood glucose, LDL cholesterol and enolase activity in T2DM mice. Had beneficial effects on lipid homeostasis, fibrosis, inflammatory markers, nephrotoxicity and cardiac hypertrophy. Down-regulates phosphoenolpyruvate carboxykinase and sterol regulatory element-binding protein-1, which are known to produce anti-diabetic effects. Induced glucose uptake and inhibited phosphoenolpyruvate carboxykinase (PEPCK) expression in vitro. Reduced neuron-specific enolase (NSE) levels and suppressed neuroinflammation in an acute spinal cord injury (SCI) model. - Chemical. CAS: 1177827-73-4 (free base). Formula: C31H43FN8O3 . HCl. MW: 594.7 . 36.5. . Cell-permeable first non-substrate analog enolase inhibitor (IC50=0.576microM). A recent study suggested no directly blocking of enolase activity in vitro and a indirect mechanism. Useful agent for immunometabolism research. The glycolysis enzymes enolase is known to have additional, non-glycolytic roles in cellular physiology, which has been termed moonlighting. Anticancer agent. Inhibits cancer cell metastasis in a zebrafish cancer cell xenograft model. Induces cell death under hypoxia and inhibits cancer cell migration and invasion by down-regulation of AKT and Bcl-xL expression. Antidiabetic agent. Reduced hyperglycemia and hyperlipidemia in mice. Reduced blood glucose, LDL cholesterol and enolase activity in T2DM mice. Had beneficial effects on lipid homeostasis, fibrosis, inflammatory markers, nephrotoxicity and cardiac hypertrophy. Down-regulates phosphoenolpyruvate carboxykinase and sterol regulatory element-binding protein-1, which are known to produce anti-diabetic effects. Induced glucose uptake and inhibited phosphoenolpyruvate carboxykinase (PEPCK) expression in vitro. Reduced neuron specific enolase (NSE) levels and suppressed neuroinflammation in an acute spinal cord injury (SCI) model.
- SMILESFC(C=C1)=CC=C1CNC2=NC(NC3=CC=C(CC(NCCOCCOCCN)=O)C=C3)=NC(NCC4CCCCC4)=N2.Cl
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- A unique small molecule inhibitor of enolase clarifies its role in fundamental biological processes: D.W. Jung, et al.; ACS Chem. Biol. 8, 1271 (2013)
- ENOblock Does Not Inhibit the Activity of the Glycolytic Enzyme Enolase: N. Satani, et al.; PLoS One 11, e0168739 (2016)
- ENOblock, a unique small molecule inhibitor of the non-glycolytic functions of enolase, alleviates the symptoms of type 2 diabetes: H. Cho, et al.; Sci. Rep. 7, 44186 (2017)
- Targeting Enolase in Reducing Secondary Damage in Acute Spinal Cord Injury in Rats: A. Haque, et al.; Neurochem. Res. 42, 2777 (2017)
