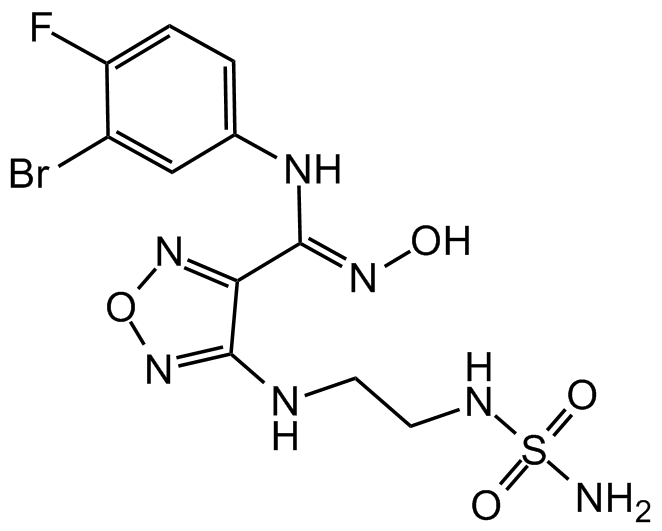
Chemical Structure
Epacadostat [1204669-58-8]

AG-CR1-3634
CAS Number1204669-58-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight438.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameEpacadostat [1204669-58-8]
- Delivery Days Customer10
- CAS Number1204669-58-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous
- Molecular FormulaC11H13BrFN7O4S
- Molecular Weight438.2
- Scientific DescriptionChemical. CAS: 1204669-58-8. Formula: C11H13BrFN7O4S. MW: 438.2. Potent and selective competitive indoleamine 2,3-dioxygenase (IDO1) inhibitor. Inhibits IDO1 in enzymatic (IC50=72nM) and in cellular tests (IC50=7.1nM), suppresses tryptophan catabolism in vivo and impedes tumor growth. Displays high selectivity over other related enzymes such as IDO2 or tryptophan 2,3-dioxygenase (TDO). Exhibits potent in vitro and in vivo immunomodulating and antineoplastic activities. By inhibiting IDO1 and decreasing kynurenine levels in tumor cells, Epacadostat increases and restores the proliferation and activation of various immune cells, including dendritic cells (DCs), NK cells and T-lymphocytes, as well as interferon (IFN) production and reduces tumor-associated regulatory T cells (Tregs). - Potent and selective competitive indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor. Inhibits IDO1 in enzymatic (IC50=72nM) and in cellular tests (IC50=7.1nM), suppresses tryptophan catabolism in vivo and impedes tumor growth. Displays high selectivity over other related enzymes such as IDO2 or tryptophan 2,3-dioxygenase (TDO). Exhibits potent in vitro and in vivo immunomodulating and antineoplastic activities. By inhibiting IDO1 and decreasing kynurenine levels in tumor cells, Epacadostat increases and restores the proliferation and activation of various immune cells, including dendritic cells (DCs), NK cells and T-lymphocytes, as well as interferon (IFN) production and reduces tumor-associated regulatory T cells (Tregs).
- SMILESO=S(NCCNC1=NON=C1/C(NC2=CC=C(F)C(Br)=C2)=N/O)(N)=O
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors: H.K. Koblish, et al.; Mol. Cancer Ther. 9, 489 (2010)
- Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity: X. Liu, et al.; Blood 115, 3520 (2010)
- Trial watch: IDO inhibitors in cancer therapy: E. Vacchelli, et al.; Oncoimmunology 3, e957994 (2014)
- Challenges in the Discovery of Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitors: U.F. Roehrig, et al.; J. Med. Chem. 58, 9421 (2015)
