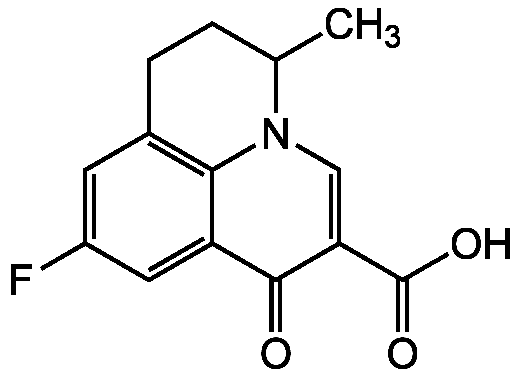
Chemical Structure
Flumequine [42835-25-6]

CDX-F0079
CAS Number42835-25-6
Product group Chemicals
Estimated Purity>97%
Molecular Weight261.3
Overview
- SupplierChemodex
- Product NameFlumequine [42835-25-6]
- Delivery Days Customer10
- CAS Number42835-25-6
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationNon-hazardous
- Molecular FormulaC14H12FNO3
- Molecular Weight261.3
- Scientific DescriptionChemical. CAS: 42835-25-6. Formula: C14H12FNO3. MW: 261.3. Synthetic. Flumequine is a fluoroquinolone synthetic chemotherapeutic antibiotic used to treat bacterial infections. Targets primarily gram negative bacteria, especially those which cause enteric infections in animals. It is used to study processes that affect mammalian chromosome and DNA unwinding at the level of gyrase/topoisomerases. It inhibits topoisomerases, which are needed for the transcription and replication of bacterial DNA. The inhibition of the topoisomerases results in strand breakage of the bacterial chromosome, supercoiling and resealing. Therefore, DNA replication and transcription is inhibited. It was also used to study hepatocarcinogenicity and DNA damage in mice and the mechanisms of quinolone resistance. - Flumequine is a fluoroquinolone synthetic chemotherapeutic antibiotic used to treat bacterial infections. Targets primarily gram negative bacteria, especially those which cause enteric infections in animals. It is used to study processes that affect mammalian chromosome and DNA unwinding at the level of gyrase/topoisomerases. It inhibits topoisomerases, which are needed for the transcription and replication of bacterial DNA. The inhibition of the topoisomerases results in strand breakage of the bacterial chromosome, supercoiling and resealing. Therefore, DNA replication and transcription is inhibited. It was also used to study hepatocarcinogenicity and DNA damage in mice and the mechanisms of quinolone resistance.
- SMILESCC1CCC2=C3N1C=C(C(O)=O)C(=O)C3=CC(F)=C2
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
