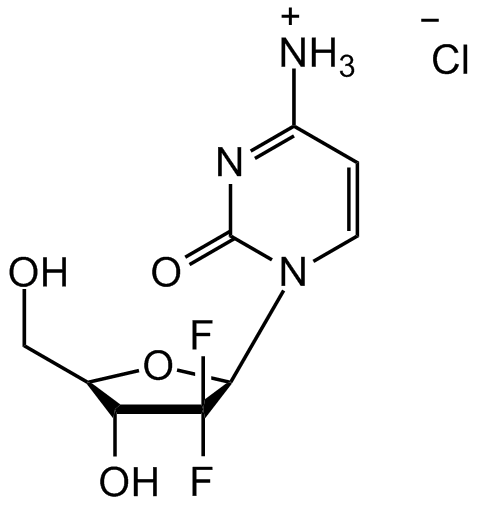
Chemical Structure
Gemcitabine hydrochloride [122111-03-9]

CDX-G0063
CAS Number122111-03-9
Product group Chemicals
Estimated Purity>98%
Molecular Weight263.20 . 36.46
Overview
- SupplierChemodex
- Product NameGemcitabine hydrochloride [122111-03-9]
- Delivery Days Customer10
- CAS Number122111-03-9
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Non-hazardous
- Molecular FormulaC9H11F2N3O4 . HCl
- Molecular Weight263.20 . 36.46
- Scientific DescriptionChemical. CAS: 122111-03-9. Formula: C9H11F2N3O4 . HCl. MW: 263.20 . 36.46. Synthetic. Widely used antitumor agent in vitro and in vivo. Deoxycytidine analog with cytotoxic effects. Inhibits DNA synthesis and induces apoptosis through inhibition of ribonucleotide reductase (R1 and R2). Following the uptake of this prodrug into cells by nucleoside transporters it is phosphorylated to its mono(dFdCMP), di(dFdCDP), and triphosphorylated(dFdCTP) forms by deoxycytidine kinase. dFdCDP and dFdCTP are reported to inhibit the activity of ribonucleotide reductase and impede DNA synthesis and repair mechanisms and induce cell death. The triphosphate form is incorporated into DNA which induces masked chain termination and cell death. By specifically inhibiting growth arrest and DNA damage inducible protein 45 a (Gadd45a), a key mediator of active DNA demethylation, inhibits repair-mediated DNA demethylation in a methylation-sensitive reporter assay at concentrations ranging from 34-134nM. This anticancer nucleoside analog inhibits the growth of HL-60 promyelocytic leukemia cells with an LC50 value of 40nM. It inhibits the growth of MX-1 mammary, CX-1, HC-1, GC3, and VRC5 colon, LX-1, Calu-6 and NCI-H460 lung, and HS766T, PaCa-2, PANC-1, and BxPC-3 pancreatic cancer tumors in mouse xenograft models (45-93% inhibition). Also shown to block mitochondrial DNA polymerase gamma. Works synergistically with other chemotherapeutic agents to enhance their cytotoxicity. Has also broad antiretroviral activity, decreasing MuLV cell infectivity, a murine AIDS model, in cell culture (EC50 = ~1.5nM) and inhibits the progression of murine AIDS in vivo at a dose of 1-2 mg/kg per day. - Widely used antitumor agent in vitro and in vivo. Deoxycytidine analog with cytotoxic effects. Inhibits DNA synthesis and induces apoptosis through inhibition of ribonucleotide reductase (R1 and R2). Following the uptake of this prodrug into cells by nucleoside transporters it is phosphorylated to its mono(dFdCMP), di(dFdCDP), and triphosphorylated(dFdCTP) forms by deoxycytidine kinase. dFdCDP and dFdCTP are reported to inhibit the activity of ribonucleotide reductase and impede DNA synthesis and repair mechanisms and induce cell death. The triphosphate form is incorporated into DNA which induces masked chain termination and cell death. By specifically inhibiting growth arrest and DNA damage inducible protein 45 a (Gadd45a), a key mediator of active DNA demethylation, inhibits repair-mediated DNA demethylation in a methylation-sensitive reporter assay at concentrations ranging from 34-134nM. This anticancer nucleoside analog inhibits the growth of HL-60 promyelocytic leukemia cells with an LC50 value of 40nM. It inhibits the growth of MX-1 mammary, CX-1, HC-1, GC3, and VRC5 colon, LX-1, Calu-6 and NCI-H460 lung, and HS766T, PaCa-2, PANC-1, and BxPC-3 pancreatic cancer tumors in mouse xenograft models (45-93% inhibition). Also shown to block mitochondrial DNA polymerase gamma. Works synergistically with other chemotherapeutic agents to enhance their cytotoxicity. Has also broad antiretroviral activity, decreasing MuLV cell infectivity, a murine AIDS model, in cell culture (EC50 = ~1.5nM) and inhibits the progression of murine AIDS in vivo at a dose of 1-2 mg/kg per day.
- SMILESOC[C@@H]1[C@@H](O)C(F)(F)[C@H](N2C=CC([NH3+])=NC2=O)O1.[Cl-]
- Storage InstructionRT
- UNSPSC12352200
