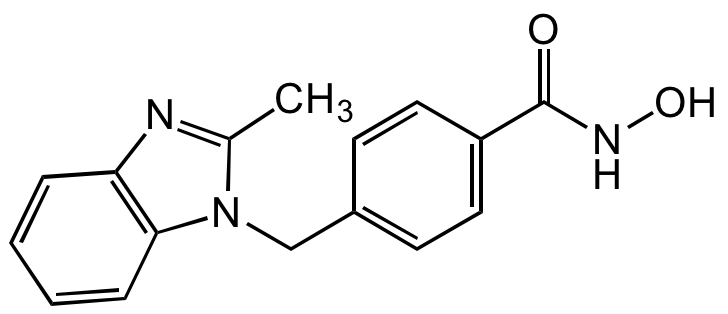
Chemical Structure
MBIMPH [1392835-53-8]

AG-CR1-3907
CAS Number1392835-53-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight281.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameMBIMPH [1392835-53-8]
- Delivery Days Customer10
- CAS Number1392835-53-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationNon-hazardous
- Molecular FormulaC16H15N3O2
- Molecular Weight281.3
- Scientific DescriptionCell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =9nM). Displays high selectivity over all other HDACs (IC50=0.1-12microM). Induces cellular alpha-tubulin, but not histone H3 hyperacetylation in Neuro-2a cells. Promotes mitochondrial transport. Shows improved kinetics and biochemical potency against HDAC6 compared to tubastatin A (Prod. No. AG-CR1-3900). HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration. - Chemical. CAS: 1392835-53-8. Formula: C16H15N3O2. MW: 281.3. Cell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =9nM). Displays high selectivity over all other HDACs (IC50=0.1-12microM). Induces cellular alpha-tubulin, but not histone H3 hyperacetylation in Neuro-2a cells. Promotes mitochondrial transport. Shows improved kinetics and biochemical potency against HDAC6 compared to tubastatin A (Prod. No. AG-CR1-3900). HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration.
- SMILESCC1=NC2=C(C=CC=C2)N1CC1=CC=C(C=C1)C(=O)NO
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Bicyclic-capped histone deacetylase 6 inhibitors with improved activity in a model of axonal charcot-marie-tooth disease: S. Shen, et al.; ACS Chemical Neuroscience (submitted) (2015)
