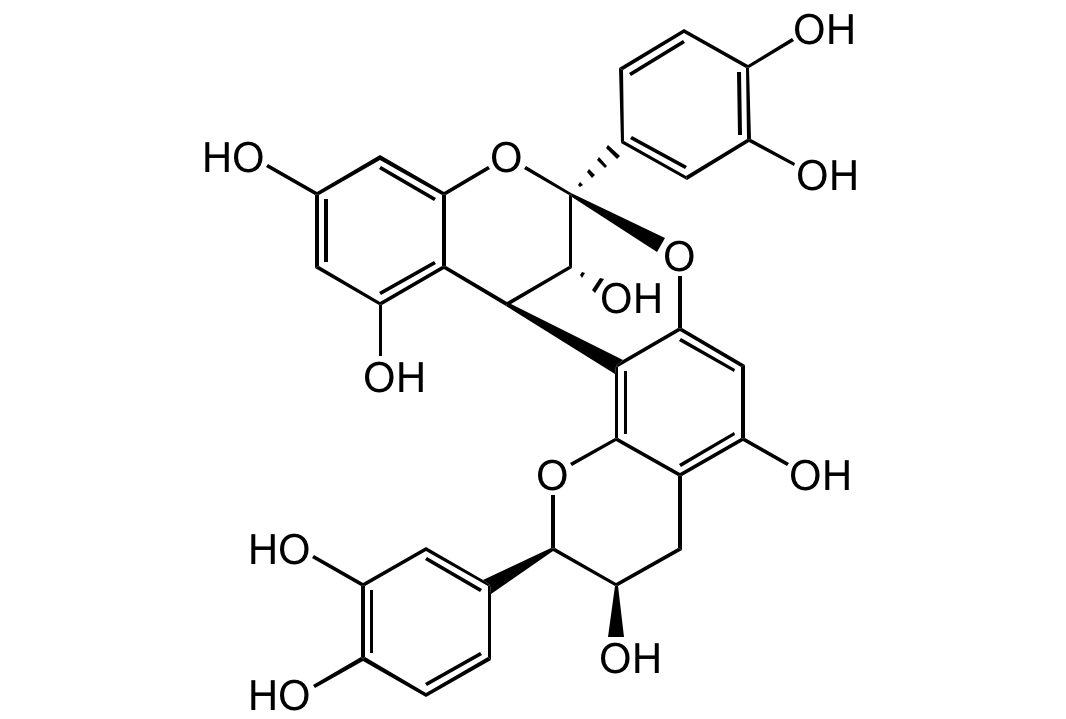
Chemical Structure
Procyanidin A2 [41743-41-3]

AG-CN2-0486
CAS Number41743-41-3
Product group Chemicals
Estimated Purity>95% (NMR)
Molecular Weight576.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameProcyanidin A2 [41743-41-3]
- Delivery Days Customer10
- CAS Number41743-41-3
- CertificationResearch Use Only
- Estimated Purity>95% (NMR)
- Hazard InformationNon-hazardous
- Molecular FormulaC30H24O12
- Molecular Weight576.5
- Scientific DescriptionA-Type proanthocyanidine. Potent antioxidant. Free radical scavenger and lipid peroxidation inhibitor. Potent low-density lipoprotein (LDL) oxidation inhibitor. Antidiabetic. Prevents islet cell apoptosis, hyperglycemia and type 2 diabetes through its antioxidant properties. Antibacterial agent. Antiviral. Shown to inhibit viral replication of canine distemper virus (CDV). Glycosyltransferase B (GTFB) inhibitor. - Chemical. CAS: 41743-41-3. Formula: C30H24O12. MW: 576.5. Isolated from grape seeds (Vitis vinifera L). A-Type proanthocyanidine. Potent antioxidant. Free radical scavenger and lipid peroxidation inhibitor. Potent low-density lipoprotein (LDL) oxidation inhibitor. Antidiabetic. Prevents islet cell apoptosis, hyperglycemia and type 2 diabetes through its antioxidant properties. Antibacterial agent. Antiviral. Shown to inhibit viral replication of canine distemper virus (CDV). Glycosyltransferase B (GTFB) inhibitor.
- SMILESOC1=CC(O)=C([C@H](C2=C(O[C@H](C3=CC(O)=C(O)C=C3)[C@H](O)C4)C4=C(O)C=C2O5)[C@@H](O)[C@@]5(C6=CC(O)=C(O)C=C6)O7)C7=C1
- Storage Instruction2°C to 8°C,-20°C
- UNSPSC12352200
References
- Phenolic constituents in the fruits of Cinnamomum zeylanicum and their antioxidant activity: G.K. Jayaprakasha, et al.; J. Agric. Food Chem. 54, 1672 (2006)
- Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity: S. Gregoire, et al.; J. Appl. Microbiol. 103, 1960 (2007)
- Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo: H. Koo, et al.; Caries Res. 44, 116 (2010)
- Doubly linked, A-type proanthocyanidin trimer and other constituents of Ixora coccinea leaves and their antioxidant and antibacterial properties: T.O. Idowu, et al.; Phytochemistry 71, 2092 (2010)
- Inhibition of viral RNA synthesis in canine distemper virus infection by proanthocyanidin A2: L. Gallina, et al.; Antiviral Res. 92, 447 (2011)
- Flavonoids from Lindera glauca blume as low-density lipoprotein oxidation inhibitors: G.W. Huh, et al.; Nat. Prod. Res. 28, 831 (2014)
- Procyanidins from the stem wood of Machilus japonica and their inhibitory effect on LDL oxidation: H.J. Park, et al.; Arch. Pharm. Res. 37, 1403 (2014)
- Chemical composition, antioxidant and antinociceptive properties of Litchi chinensis leaves: R.C. Castellain, et al.; J. Pharm. Pharmacol. 66, 1796 (2014)
- Preventive effects of procyanidin A2 on glucose homeostasis, pancreatic and duodenal homebox 1, and glucose transporter 2 gene expression disturbance induced by bisphenol A in male mice: A. Ahangarpour, et al.; J. Physiol. Pharmacol. 67, 243 (2016)
