Minimizing False Positives/Negatives with PBL’s Kits (no.1)
PBL’s VeriKine-HS ELISA kits ensure reliable results across healthy and disease-patient samples by optimizing sensitivity, specificity, and matrix compatibility to minimize assay interference.
Performance Characterization of Human IL-22 ELISA Kit
The Biological Role of IL-22
Interleukin-22 (IL-22) is a multifunctional cytokine belonging to the IL-10 family, produced by a diverse range of immune cells including TH1, TH17, TH22 T cells, natural killer T (NKT) cells, type 3 innate lymphoid cells (ILC3), neutrophils, and macrophages. Unlike many cytokines that act on immune cells, IL-22 primarily targets non-hematopoietic cells such as epithelial and stromal cells. It plays a dual role in immune regulation—promoting tissue protection and regeneration (e.g., enhancing epithelial barrier integrity and hepatocyte survival) while also contributing to inflammation under certain pathological conditions.
IL-22 in Disease Pathogenesis
IL-22 is increasingly recognized as a key player in the pathogenesis of several autoimmune and inflammatory diseases, including Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA), and Psoriasis. It is often considered a hallmark of IL-17-driven immune responses, acting synergistically with IL-17 to amplify inflammatory signaling in target tissues.
Assay Design and Specificity
To support accurate and reproducible measurement of this biologically significant cytokine, PBL’s VeriKine High Sensitivity Human IL-22 ELISA is engineered to detect free IL-22 with high specificity and sensitivity. The assay uses a recombinant IL-22 standard expressed in HEK-293 cells, closely mimicking the native human protein. Importantly, the assay’s performance suggests that it selectively measures free IL-22, as high concentrations of IL-22 binding protein (IL-22BP) inhibit detection, indicating minimal cross-reactivity with bound forms.
Kit Sensitivity and Validation
PBL‘s kit features a calibration range of 0.78–50 pg/ml and a limit of quantitation (LOQ) below 1 pg/ml, making it suitable for detecting low-abundance IL-22 in complex biological matrices. It has been rigorously validated for use with healthy and diseased human serum, plasma, and tissue culture media, consistently quantifying endogenous IL-22 in the expected 1–10 pg/ml range in healthy donors. Additionally, 100% of tested disease-state samples yielded quantifiable results.
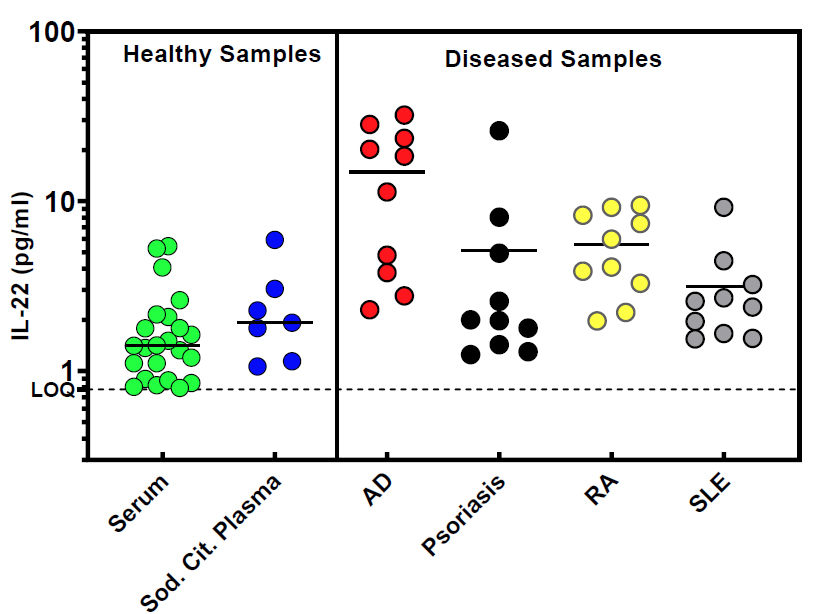
Performance Validation
Performance validation includes:
- Strong correlation with Quanterix ultra-sensitive technology (r = 0.83), demonstrating cross-platform consistency.
- Excellent dilution linearity and precision, confirming the assay’s robustness.
- High recovery rates for spiked IL-22 in serum, plasma, and tissue culture media, supporting its reliability in complex sample types.
Research Applications
Together, these features make PBL’s IL-22 ELISA a powerful tool for researchers studying IL-22 biology, immune regulation, and its role in autoimmune and inflammatory diseases.
Real Sample Performance of PBL’s High Sensitivity Human IL-22 ELISA Kit:
Download PBL’s poster to see how the High Sensitivity Human IL-22 ELISA Kit performs in real samples.

 Find products for your research
Find products for your research