Secondary Antibodies Are of Primary Importance
When your blot looks noisy or your fluorescent image fades, don’t blame the primary antibody. The real culprit is often the secondary. ICL shows you how to choose wisely and watch faint bands pop clear.
ICL: The Critical Role of Host Cell Protein Detection and HCP analysis in host cell protein quantification in Biotherapeutic Manufacturing
Host Cell Protein ELISA is a laboratory technique used to detect and quantify proteins originating from host cells in biopharmaceutical production, particularly in the context of host cell protein analysis. It utilizes specific antibodies that bind to the target proteins, allowing for accurate measurement. This process ensures product safety and quality, crucial in drug development and manufacturing.
As biotherapeutics continue to revolutionize the treatment of cancer, autoimmune diseases, and rare genetic disorders, the standards for product purity and safety have never been higher. Unlike small-molecule drugs, biotherapeutics are produced using living cell lines or cells, typically mammalian expression systems like Chinese Hamster Ovary (CHO) cells, which introduce new challenges into the manufacturing process. Chief among these challenges is the presence of host cell proteins (HCPs), which are process-related protein impurities: unwanted residual proteins, considered contamination, that are naturally produced by the host cells during recombinant protein expression, potentially affecting the quality of the final product.
Even after extensive purification, trace levels of HCPs can remain in the final drug product, posing serious risks to both patient safety and potential toxicity and therapeutic efficacy. These contaminants can trigger immune reactions, interfere with drug stability, or degrade critical excipients and adjuvants. As a result, regulatory agencies worldwide, including the FDA and EMA, require robust detection and quantification of HCPs throughout the development, scale-up, and manufacturing of biologic drugs.
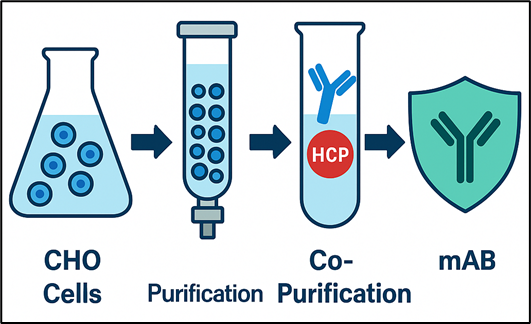
Detecting and minimizing HCPs is therefore a foundational aspect of quality control in biopharmaceutical manufacturing. Accurate HCP measurement, as conducted in host cell protein ELISA’s not only supports regulatory compliance from regulatory bodies but also enables drug developers to fine-tune their purification processes, reduce variability, and ensure consistent product performance across production batches.
Chinese Hamster Ovary (CHO) cells are the gold standard for producing monoclonal antibodies and other biologics due to their adaptability and human-like post-translational modifications. However, CHO cells also generate hundreds of endogenous proteins that can co-purify with therapeutic products, risking:
Regulatory agencies worldwide require thorough characterization and quantification of HCPs, especially during late-stage development and manufacturing.
Generic host cell protein ELISA assays, including the host cell protein immunosorbent assay (ELISA), are often used in early process development and process control but may fail to detect specific immunogenic or process-altering proteins. As such, there is a growing industry shift toward individual HCP assays that allow for precise monitoring of high-risk proteins like PLBL2 (Phospholipase B-Like 2) and NUCB2 (Nucleobindin-2), where advanced techniques like the mass spectrometer can enhance detection accuracy in the production of biologics.
ICL was among the first to commercialize ELISA kits using in-house antibodies that specifically detect these problematic CHO-derived proteins. Our proprietary assays help ensure patient safety, product stability, and regulatory success.
We offer a full line of CHO-specific antibodies, ELISA kits, and purified proteins for targeted HCP detection, including:
ICL’s antibodies and ELISA kits are developed and validated in-house at our Oregon-based facility. Our experience in producing reagents for the IVD and research markets ensures:
Whether you’re optimizing upstream cell culture or validating downstream purification, ICL provides the tools you need to meet compliance standards and avoid costly setbacks.
Explore ICL’s full range of CHO-specific host cell protein reagents and ELISA kits, including products for:
ICL’s ELISA kits and antibodies for detecting host cell proteins like PLBL2 and NUCB2 are trusted by scientists in both academic research and commercial biopharmaceutical development. Our reagents have been cited in recent peer-reviewed publications:
Lakatos, D., Buffer System Improves the Removal of Host Cell Protein Impurities in Monoclonal Antibody Purification. Biotechnology and Bioengineering. 2024.
Falkenberg H, Mass spectrometric evaluation of upstream and downstream process influences on host cell protein patterns in biopharmaceutical products. Biotechnol Prog. 2019.
Dolan ME, First site-specific conjugation method for native goat IgG antibodies via glycan remodeling at the conserved Fc region. Antib Ther. 2024
The landscape of host cell protein (HCP) analysis is rapidly evolving, driven by regulatory pressure, advances in analytical technologies, and the increasing complexity of biologic drugs. While traditional generic HCP ELISAs remain a staple for early-phase development, there is a clear shift toward targeted, product-specific assays that offer greater sensitivity and specificity for high-risk impurities such as PLBL2 and NUCB2. In parallel, orthogonal techniques like mass spectrometry and 2D-DIGE are being integrated to complement ELISA data, offering deeper insight into HCP profiles across upstream and downstream processes. As biomanufacturers aim for sub-ppm detection and process consistency, the demand for high-quality, validated reagents—especially those capable of detecting immunogenic or degradation-prone HCPs—continues to grow.
Contact us for technical data, custom assay development, or bulk pricing options.
We gladly support you by keeping you updated on our latest products and the developments around our services.