GeneTex’s “5 + 1” pillar antibody validation process
GeneTex’s production focus is dedicated to the creation of novel recombinant monoclonal antibodies validated using enhanced protocols defined by the International Working Group for Antibody Validation (IWGAV).
Over the last decade, studies published in many of the top scientific journals have clearly described the significant impact of poorly validated antibody reagents on the reproducibility of biomedical research. The unfortunate reality is that the commercial antibody market is compromised by a substantial subset of products that have been demonstrated to be nonspecific. This means that there are two major issues that must be addressed in order to improve the overall quality of the commercial antibody market and therefore the integrity and reproducibility of the biomedical research dependent on antibodies. The first is more widespread adoption of production platforms that emphasize recombinant antibody technology while the second involves incorporation of improved and more coherent validation strategies. GeneTex is making major strides in both of these endeavors.
As was discussed in a prior news feature, GeneTex has already shifted completely to the production of recombinant monoclonal antibodies (figure 1). Fully recombinant antibodies are defined by their primary sequence, their consistent performance and supply (issues that plague traditional polyclonals and hybridoma-based monoclonals with regard to lot-to-lot variability), and their ability to be engineered (i.e., backbone switching) to meet researcher requirements. The numerous advantages of recombinant antibodies may even evoke more favorable impressions by grant review and journal editorial committees.
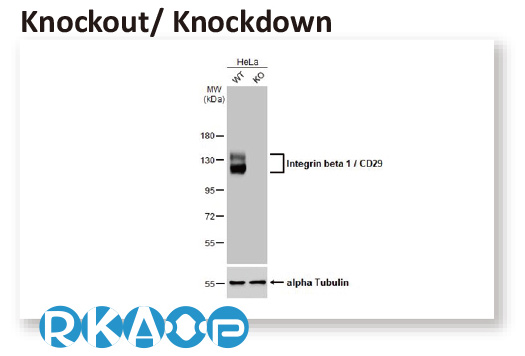
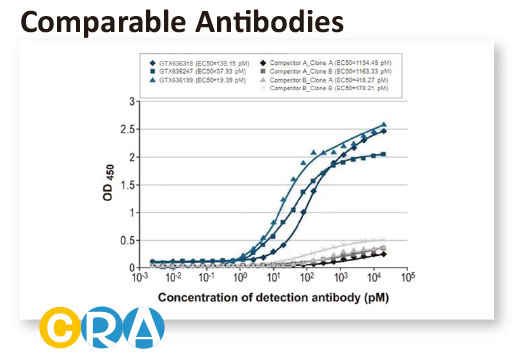
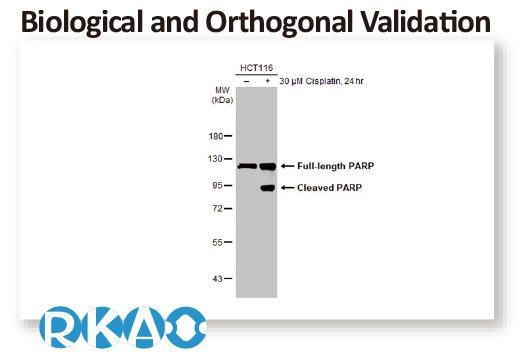
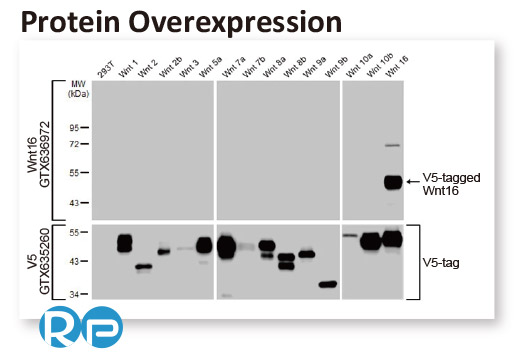
In conjunction with the pivot to recombinant antibody production, GeneTex’s second commitment to antibody reliability is its incorporation of enhanced validation protocols, based on the IWGAV proposal mentioned above, into its manufacturing and quality assurance (QA) workflow (1). This involves evaluation of each antibody using at least one, and preferably more, of five validation “pillars” defined in the IWGAV study (1). GeneTex’s version of the IWGAV plan follows the five standard strategies and includes (1) Knockout/Knockdown; (2) Comparable Antibodies; (3) Immunoprecipitation followed by Mass Spectrometry (IP/MS); (4) Biological and Orthogonal Validation; and (5) Recombinant Protein Expression (Figure 2). The company refers to this as its “5+1” pillar plan when referencing new recombinant antibody production, with the “+1” designation referring to the pre-validation that is inherent in the application-specific assessment that occurs with clone selection during the recombinant antibody production process (Table 1 and the product validation examples below). With an extensive product catalog, GeneTex is working to replace its traditional polyclonals with new recombinants as well as developing novel antibodies against additional targets. It is also striving to apply IWGAV five pillar validation to other products already in the catalog. The task is daunting, but GeneTex is making some progress as noted in a CiteAb blog from October, 2022 (2).
Antibody fidelity, application-dependent reliability, and performance and supply consistency are crucial for ensuring research integrity. GeneTex joins other reputable companies in making antibodies that researchers can trust.
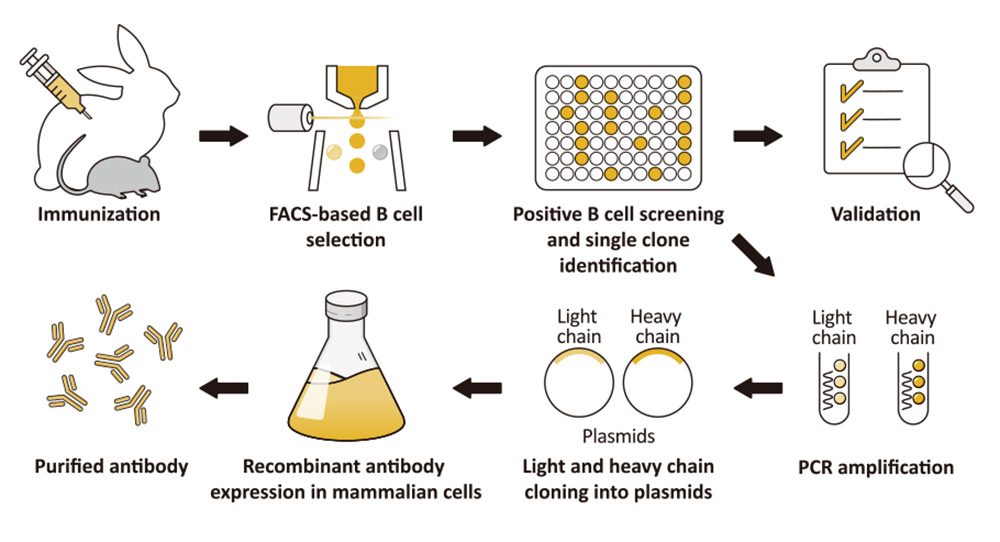

Table 1. GeneTex’s “5+1” pillar plan for recombinant antibody validation.







Product validation examples
| NFkB p105 antibody [HL1784] (GTX637436) |
| Galectin 3 antibody [HL1878] (GTX637627) |
| ZO-1 antibody [HL1133] (GTX636399) |
| ACE2 antibody [HL1092] (GTX636265) |
| BrdU antibody [HL1111] (GTX636326) |
| alpha Synuclein antibody [HL1242] (GTX636641) |
| RAS (G12D Mutant) antibody [HL10] (GTX635362) |
| Bax antibody [HL236] (GTX635715) |
| GRK2 (phospho Ser670) antibody [HL1035] (GTX635875) |
| AKT1 antibody [HL1142] (GTX636413) |
| PD-L1 antibody [HL1041] (GTX635975) |
| RAI3 antibody [HL1864] (GTX637589) |
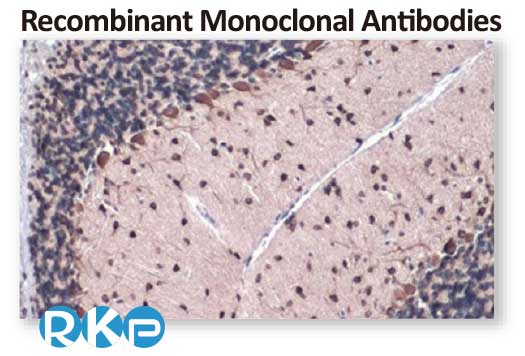
Gli1 antibody [HL247] (GTX635619)
; Application: Immunohistochemical analysis; Sample: Paraffin-embedded mouse cerebellum; Dilution: 1:50
Antigen Retrieval: Citrate buffer, pH 6.0, 15 min
| Iba1 antibody [HL22] (GTX635363) |
| Vimentin antibody [HL1506] (GTX636980) |
| EpCAM antibody [HL1339] (GTX636759) |
References
- Uhlen M, Bandrowski A, Carr S, et al. A proposal for validation of antibodies. Nat Methods. 2016;13(10):823-827.
- Williams R. Supplier antibody validation: how much progress has been made? CiteAb Blog.

 Find products for your research
Find products for your research