The Complete Guide to Host Cell Protein ELISA
ICL: The Critical Role of Host Cell Protein Detection and HCP analysis in host cell protein quantification in Biotherapeutic Manufacturing
ICL’s PLBL2 reference standard for CHO‑derived host cell proteins delivers broader sequence coverage and PTM data than the USP PLBL2 ARM for reliable HCP analysis
Host Cell Proteins (HCPs) remain one of the most persistent challenges in biopharmaceutical manufacturing. Among them, phospholipase B-like 2 (PLBL2) from CHO cells is notorious for co-purifying with monoclonal antibodies and triggering immunogenic responses. A recent study published by Michael Dolan and colleagues, published in Biotechnology and Bioengineering offers the most comprehensive characterization of PLBL2 to date, revealing its extraordinary complexity and significant implications for downstream processing.
This characterization underscores why PLBL2 has become a focal point for regulatory scrutiny and process optimization. Its structural complexity and tendency to resist clearance through conventional purification steps highlight the limitations of generic HCP assays, which often fail to detect such problematic species. As biopharmaceutical pipelines grow more diverse, the need for orthogonal and protein-specific strategies becomes critical, not only to ensure product safety but also to streamline comparability studies and accelerate timelines. Targeted tools, such as protein-specific ELISAs, can provide actionable insights into clearance efficiency and help mitigate immunogenicity risks before they escalate into regulatory hurdles.
The research team employed site-specific antibody immobilization and affinity enrichment to isolate PLBL2 from pembrolizumab, enabling deep structural analysis. Here’s what they found:

These findings underscore why PLBL2 resists clearance during standard purification steps and why precise detection and quantitation are critical for patient safety and regulatory compliance.
ICL offers high-purity recombinant CHO PLBL2 and validated antibodies that enable accurate detection and quantitation of this challenging impurity. Here’s why our protein sets the benchmark:
The recent study revealed critical gaps in the USP ARM for PLBL2. Here’s why ICL’s PLBL2 (Cat# AG65-0365) protein sets the benchmark:
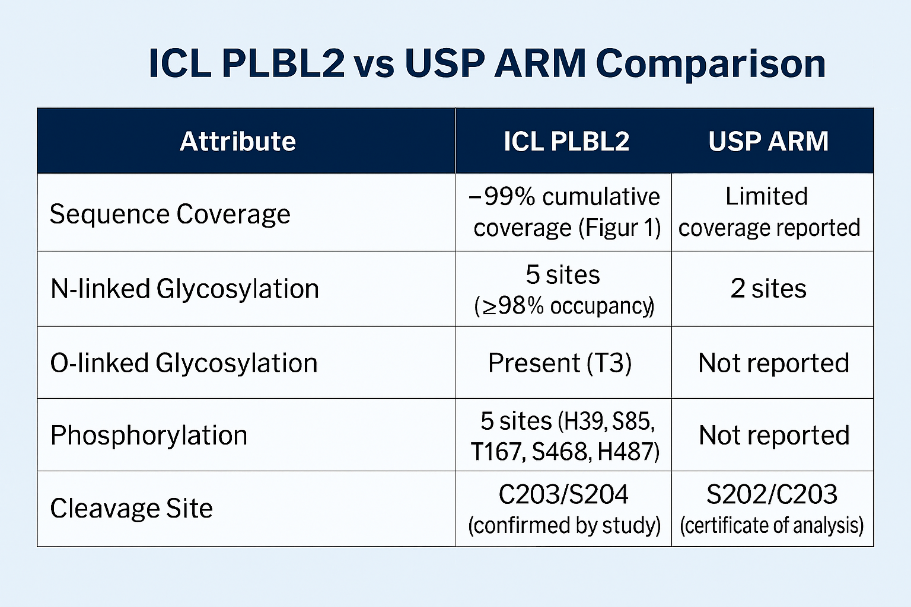
Bottom line: When precision matters, start with a reference material that reflects the true complexity of PLBL2.
Why This Matters: Accurate reference materials drive confidence in HCP detection and clearance strategies. Using a protein that mirrors endogenous PLBL2 complexity ensures your assays meet regulatory expectations and mitigate immunogenic risk.
When regulatory expectations demand confidence in your HCP analysis, starting with the right reference material is non-negotiable. ICL’s PLBL2 protein provides that confidence.
Want to learn more about how ICL can support your HCP strategy? Contact us today or read our article “The Complete Guide to Host Cell Protein ELISA” for a broader overview of HCP detection, host cell protein ELISA, and targeted HCP assays.
You can also explore ICL’s PLBL2 resources here: https://www.icllab.com/host-cell-proteins.html
We gladly support you by keeping you updated on our latest products and the developments around our services.