The SARS-CoV-2 Omicron (B.1.1.529) variant
Our suppliers provide tools to support the study of the new Omicron variant. Check out more, what Bio-Connect offers for your Covid research.
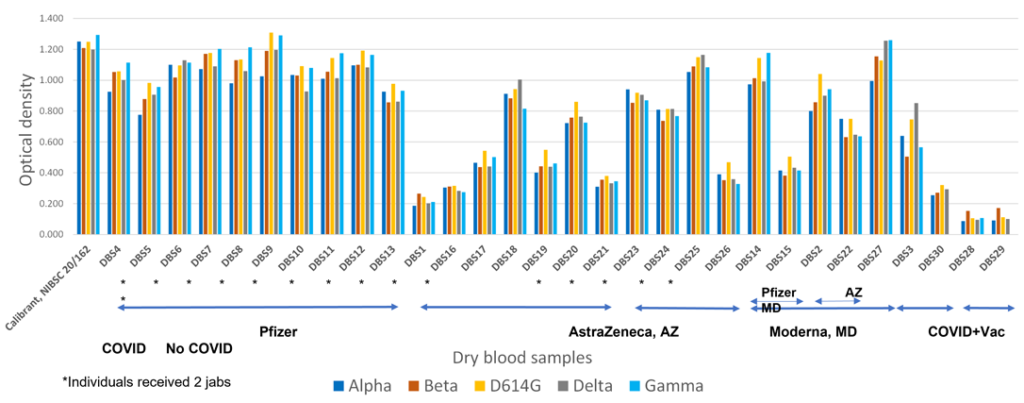
Novel COVID-19 IgG and IgM ELISA assays by Biorbyt, providing sensitive and reliable measurements of specific antibodies as a result of COVID-19 infections and vaccination programmes.
Biorbyt and Daresbury Proteins are excited to announce the launch of novel COVID-19 IgG and IgM ELISA assays, providing sensitive and reliable measurements of specific antibodies as a result of COVID-19 infections and vaccination programmes.
They manufacture trimeric Spike proteins — the most valuable COVID-19 antigen — ensuring the highest sensitivity of the resulting test. They are produced in human cell culture and therefore are the exact match of the current vaccines, providing the best tool to assess the efficacy of those vaccines.
Uniquely expressed large MW proteins with extensive post-translational modifications via codon optimised sequences in proprietary expression plasmids.
Super stable trimeric Spikes as abundant capture antigens ensuring the most faithful detection of circulating specific anti-SARS-CoV-2 antibodies in recovered or vaccinated people.
The COVID-19 ELISA IgG test is available in two formats:
Contact us for more information or quotes.
We gladly support you by keeping you updated on our latest products and the developments around our services.