Biosynth - the new brand for Biosynth Carbosynth
Biosynth Carbosynth and their recent acquisitions vivitide and Pepscan have rebranded to Biosynth.
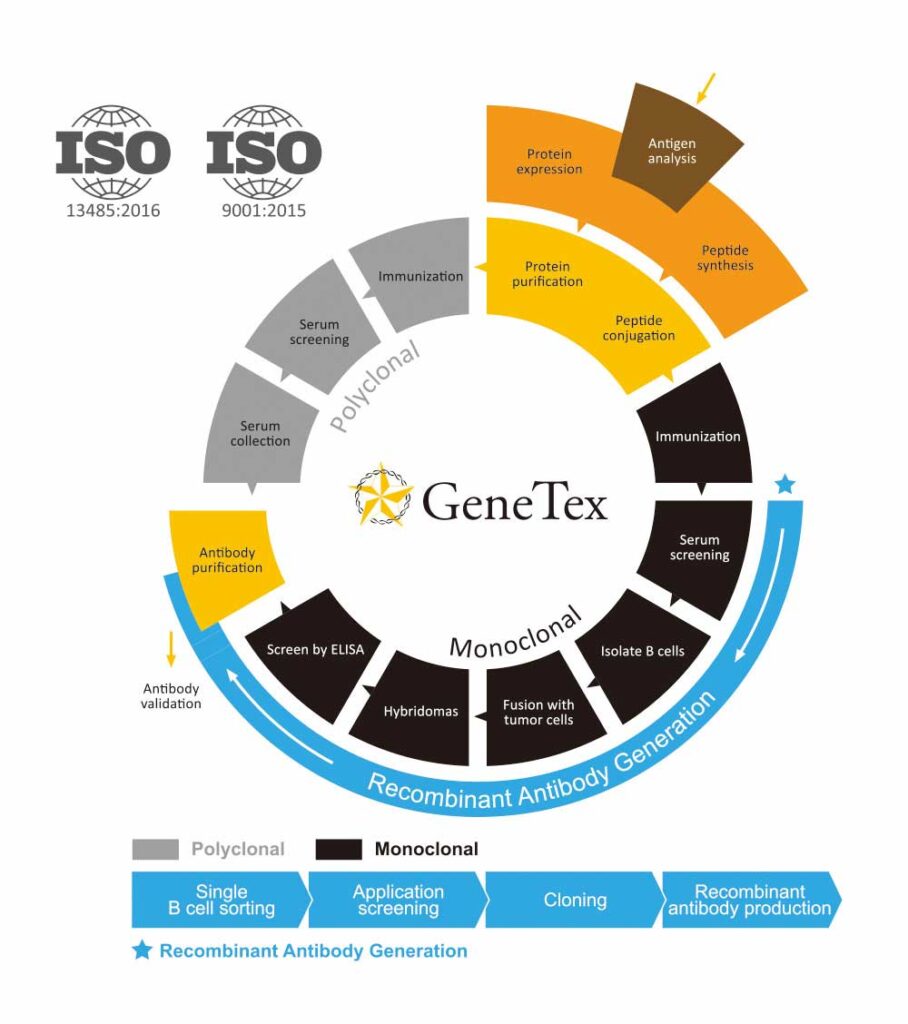
GeneTex’s antibodies and reagents are manufactured to standards outlined in Quality Management Systems (QMS) ISO 9001:2015 and ISO 13485:2016 and are supported by comprehensive validation and customer service.
GeneTex creates reagents for biomedical research, assay development, and in vitro diagnostics. The company operates state-of-the-art facilities to provide products generated through refined development and quality assurance programs, international collaboration with leading researchers, and scrupulous scientific rigor.
Since its inception in 1997 in San Antonio, Texas, GeneTex’s goal has been to offer researchers thoroughly-characterized antibodies and reagents to facilitate and expedite their scientific discoveries. This began with a series of well-published antibodies generated in the laboratories of the company’s founders, all of whom are distinguished researchers and/or clinicians.
As GeneTex sought to widen its scope, it established in 2007 a new manufacturing facility in Taiwan dedicated to the production of conventional polyclonal and mouse hybridoma monoclonal antibodies. This allowed a rapid increase in the number of antibodies available for detection of targets across the spectrum of biomedicine. Antibodies were evaluated for performance in at least three different applications, generally including western blot (WB), immunocytochemistry (ICC/IF), and immunohistochemistry (IHC). Additional applications, such as immunoprecipitation (IP), chromatin immunoprecipitation (ChIP), and flow cytometry (FCM), were assessed depending on the antibody.
Subsequent to the U.S. office’s relocation to Irvine, CA in 2009, GeneTex’s leadership made the decision to concentrate its productive capacity on the creation of recombinant rabbit antibodies. This change signaled both another milestone for the company’s growth as well as an acknowledgement of the widely reported reproducibility issues associated with conventional polyclonal and monoclonal antibodies. This expansion required the establishment of a cutting-edge recombinant antibody facility as well as additional laboratory space to accommodate enhanced validation protocols that include CRISPR-mediated knockout cells and lysates.
Attaining QMS ISO 9001:2015 and ISO 13485:2016 was a natural progression for GeneTex as it continues to increase its inventory of recombinant antibodies to complement its large catalog of reagents. While offering academic scientists the well-known benefits of recombinant technology, it also allowed GeneTex to explore the diagnostics market. The SARS-CoV-2 (COVID-19) pandemic coincided with these efforts and the company quickly produced a broad catalog of recombinant antibodies suitable for both academic and industrial applications. A similar strategy is being employed to develop antibodies for other viruses, including various flaviviruses, influenza, respiratory syncytial virus (RSV), and other pathogenic human and veterinary pathogens.
GeneTex remains positioned to continue to support academic and diagnostic biomedicine with the hope of contributing meaningfully to improving human health.
We gladly support you by keeping you updated on our latest products and the developments around our services.