Prolytix's Platelet Alpha-Granule Proteins
Explore Prolytix’s high-quality platelet alpha-granule proteins—essential for coagulation, wound healing, and research. Discover their unique functions, purity, and custom options for your scientific needs.
Discover high-purity hemoglobin and hemopexin proteins by Prolytix for research. Learn about their structure, function, and applications.
Hemoglobin is a protein found in the red blood cells of vertebrates that plays an essential role in transporting oxygen (O2) from the lungs to the tissues, and carrying carbon dioxide (CO2), a waste product, back to the lungs for removal. It binds to O2 in the lungs, forming oxyhemoglobin, and releases it in tissues where O2 levels are low. Hemoglobin also helps maintain the blood’s pH balance by carrying CO2, which is then exhaled (1).
Hemoglobin is composed of four polypeptide chains arranged in a tetrameric structure; two α chains and two β chains in adults (known as HbA). Each of the four subunits has a heme group that contains an iron atom (Fe2+) at its center that binds one O₂ molecule, enabling a single hemoglobin molecule to carry four O2 molecules. O2 binding is allosteric and the binding/release of oxygen by one subunit facilitates the binding/release of oxygen from the other subunits (1).
Cell-free hemoglobin is elevated after trauma and in other disease states that result in excessive red blood cell hemolysis. Cell-free hemoglobin degrades into globin and free heme, a potent pro-oxidant and pro-inflammatory agent (2). The plasma glycoprotein hemopexin (β-1B-glycoprotein) binds with high affinity to heme (Kd <1pM) and can extract heme from hemoglobin (3). Hemopexin interacts directly with hemoglobin’s β-chains, and molecular docking and peptide mapping reveal five binding regions (Hb1–Hb5) on hemopexin, with shared sequences (e.g., RLHIMAGRRL and KSGAQATWTE) mediating both heme and hemoglobin binding (4). Histidine residues (e.g., H105, H293) in hemopexin’s heme-binding regions coordinate iron, while serine residues (e.g., S387) may facilitate heme extraction from hemoglobin (3). Under high heme loads, hemopexin can bind heme at a 2:1 ratio (2), enhancing its scavenging capacity. The heme-hemopexin complex binds to LRP/CD91, a receptor on the surface of hepatocytes and macrophages (5). This receptor mediates the internalization of the complex leading to heme degradation and the production of bilirubin. This process plays a crucial role in iron homeostasis and protection from iron-induced oxidative damage (4). Elevated levels of hemopexin are often seen during acute-phase responses to inflammation or injury.
Prolytix now carries purified hemoglobin (Catalog # HHB-1000) and hemopexin (Catalog # HPX-1000) for your research needs. Hemoglobin is isolated from human red blood cells. Its concentration is determined using a commercially available colorimetric assay. Its purity by SDS-PAGE is ≥95%. Custom formulations including lyophilization are available. Please contact your account manager for more information.
Hemopexin is isolated from human plasma using affinity chromatography. Its concentration is assessed at 280 nm (E280 nm, 1%, 1 cm = 19.7). Its purity by SDS-PAGE is ≥95%. Its ability to bind heme is assessed spectrophotometrically. Purified hemopexin is provided in 0.01M KH2PO4, 0.03 M Na2HPO4•7H2O, 0.15 M NaCl (PBS), pH 7.4, but custom formulations including lyophilization are available.
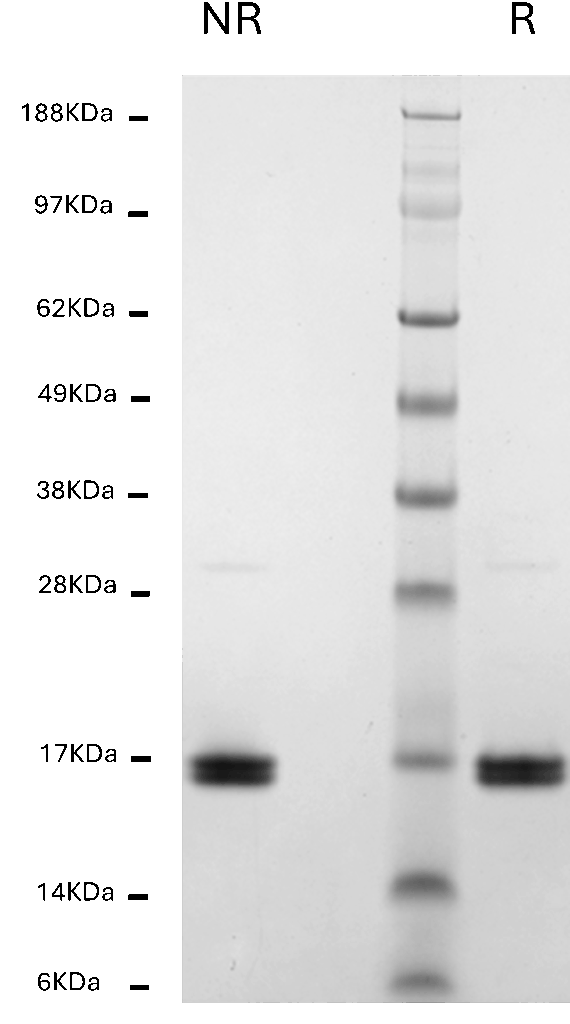
Novex 4-12% Bis-Tris Gel
Human Hemoglobin, 1 µg per lane
NR = non-reduced; R = reduced
MW Standards
Myosin (188 kDa), Phosphorylase B (98 kDa), BSA (62 kDa), Glutamic Dehydrogenase (49 kDa), Alcohol Dehydrogenase (38 kDa), Carbonic Anhydrase (28 kDa), Myoglobin Red (17 kDa), Lysozyme (14 kDa), Aprotinin (6 kDa)
| Localization | Red blood cells |
| Plasma Concentration | Whole blood, 150g/L Red blood cells, 330 g/L |
| Molecular Weight | 64,650 Da (tetramer); 16,000 Da (monomer) |
| Structure | Hemoglobin A (HbA): Tetramer containing two alpha subunits and two beta subunits (α2β2). Other tetrameric variants exist |
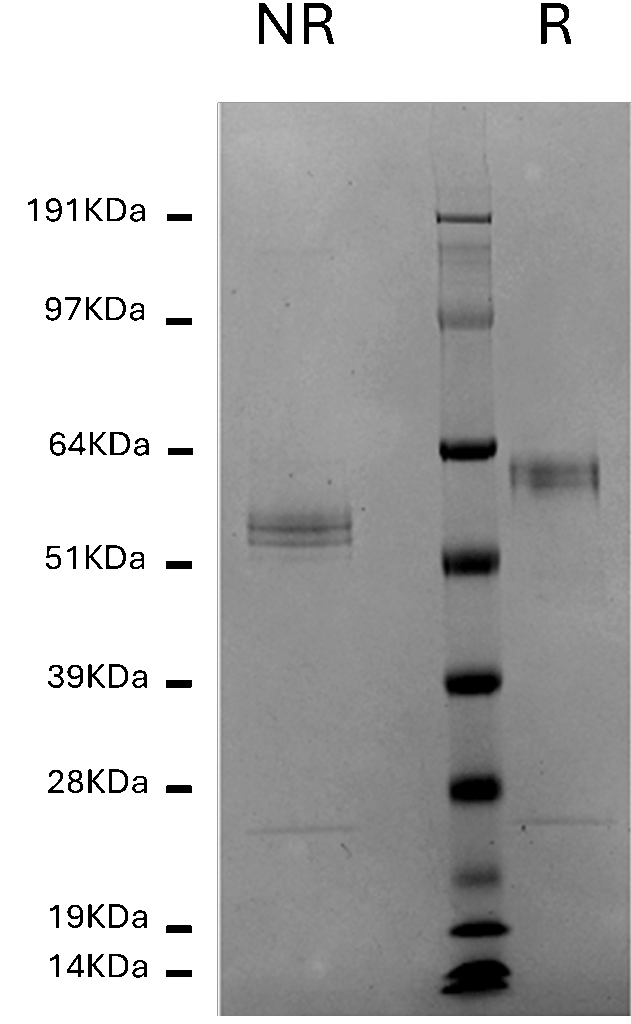
Novex 4-12% Bis-Tris Gel
Human Hemopexin, 1 µg per lane
NR = non-reduced; R = reduced
MW Standards
Myosin (191 kDa), Phosphorylase B (97 kDa), BSA (64 kDa), Glutamic Dehydrogenase (51 kDa), Alcohol Dehydrogenase (39 kDa), Carbonic Anhydrase (28 kDa), Myoglobin Red (19 kDa), Lysozyme (14 kDa)
| Localization | Plasma |
| Plasma Concentration | 0.5 – 1.0 g/L |
| Molecular Weight | 58,000 Da (SDS-PAGE) |
| Structure | Single polypeptide chain; 6 carbohydrate side chains: O-linked Thr-1, N-linked Asn-41, Asn-164, Asn-217, Asn-223, and Asn-430 |
We gladly support you by keeping you updated on our latest products and the developments around our services.