LymphoTrack® Dx Assays
NGS Analysis of Hematology-Oncology Clonality Testing
The only CE-IVD marked assay for selection of acute myeloid leukemia (AML) patients eligible for treatment with midostaurin or gilteritinib fumarate.
The LeukoStrat® CDx FLT3 Mutation Assay from Invivoscribe enables laboratories and physicians to support patients with local access to high-quality, diagnostic tests that improve treatment.
| LeukoStrat® CDx FLT3 Mutation Assay (K-412-0291) |
| LeukoStrat® CDx FLT3 Mutation Assay Software (K-412-0281) |
The LeukoStrat® CDx FLT3 Mutation Assay is a PCR-based in vitro diagnostic test designed to detect internal tandem duplications (ITD) and tyrosine kinase domain (TKD) mutations D835 and I836 in the FLT3 gene in genomic DNA extracted from mononuclear cells obtained from peripheral blood or bone marrow aspiratesof patients diagnosed with acute myelogenous leukemia (AML).
In regions where midostaurin is available, the assay is used as an aid in the assessment of patients with AML for whom RYDAPT®(midostaurin) treatment is being considered.
In regions where gilteritinib fumarate is available, the assay is used as an aid in the assessment of patients with AML for whom XOSPATA® (gilteritinib fumarate) treatment is being considered.
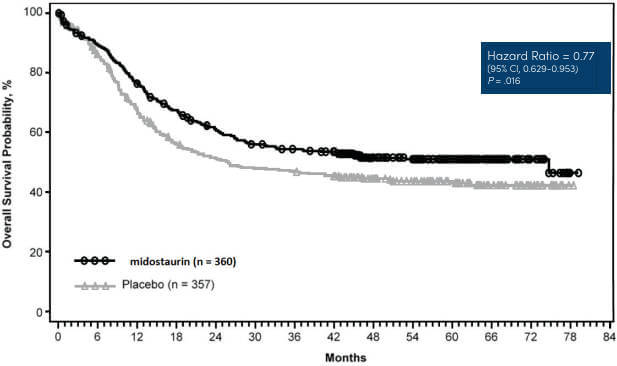
The safety and efficacy of the LeukoStrat® CDx FLT3 Mutation Assay was assessed during a bridging study, corresponding to the Phase III CPKC412A2301 (RATIFY) clinical study of midostaurin in newly diagnosed AML patients with FLT3 mutations.
European Commission approval of gilteritinib is based on Phase 3 ADMIRAL trial results which investigated gilteritinib versus salvage chemotherapy in patients with relapsed or refractory FLT3mut+ AML. The ADMIRAL study demonstrated that gilteritinib resulted in a statistically significant improvement in median overall survival (9.3 months) compared to salvage chemotherapy (5.6 months) when patients were selected with the LeukoStrat CDx FLT3 Mutation Assay.
We gladly support you by keeping you updated on our latest products and the developments around our services.