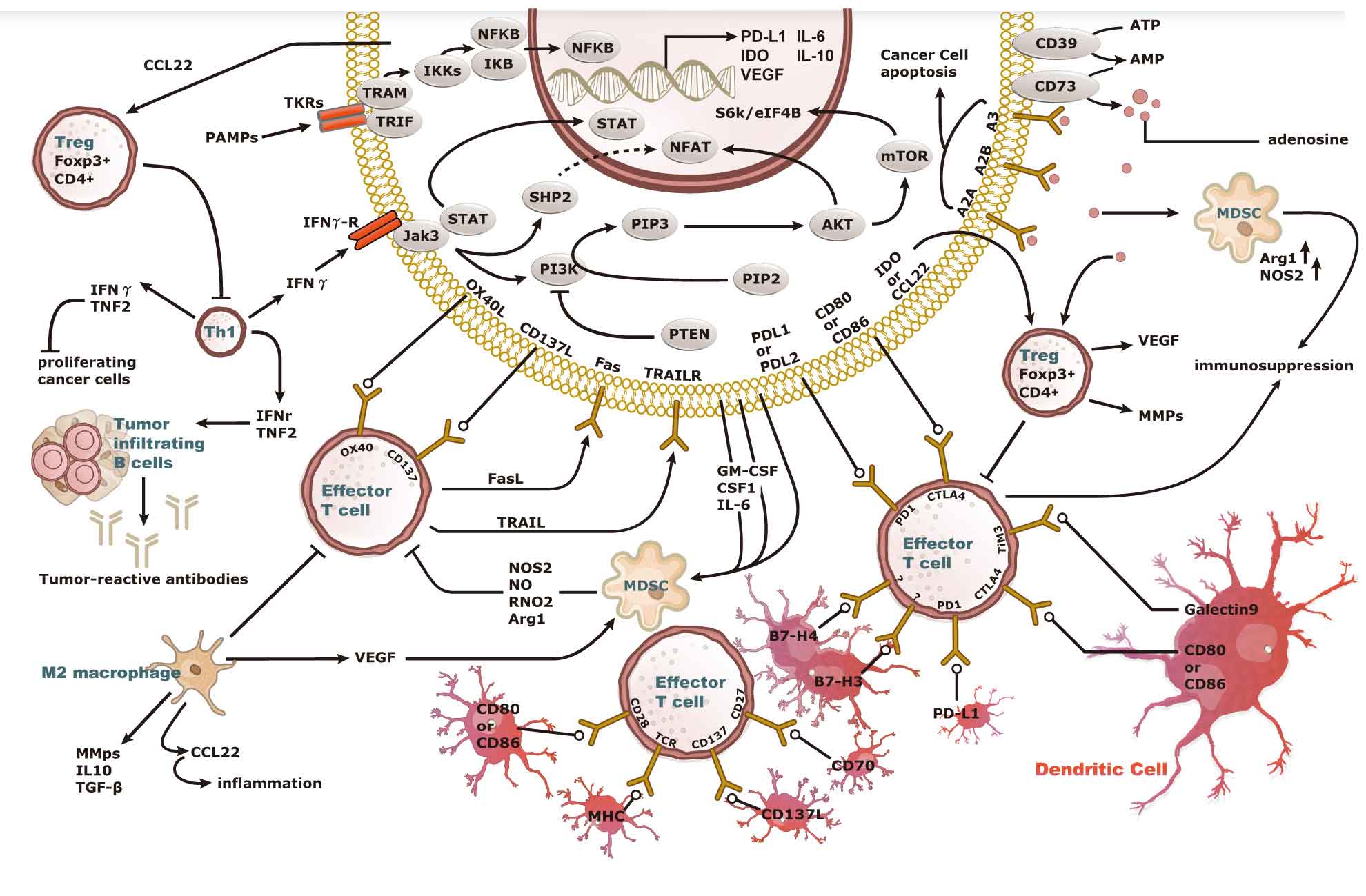
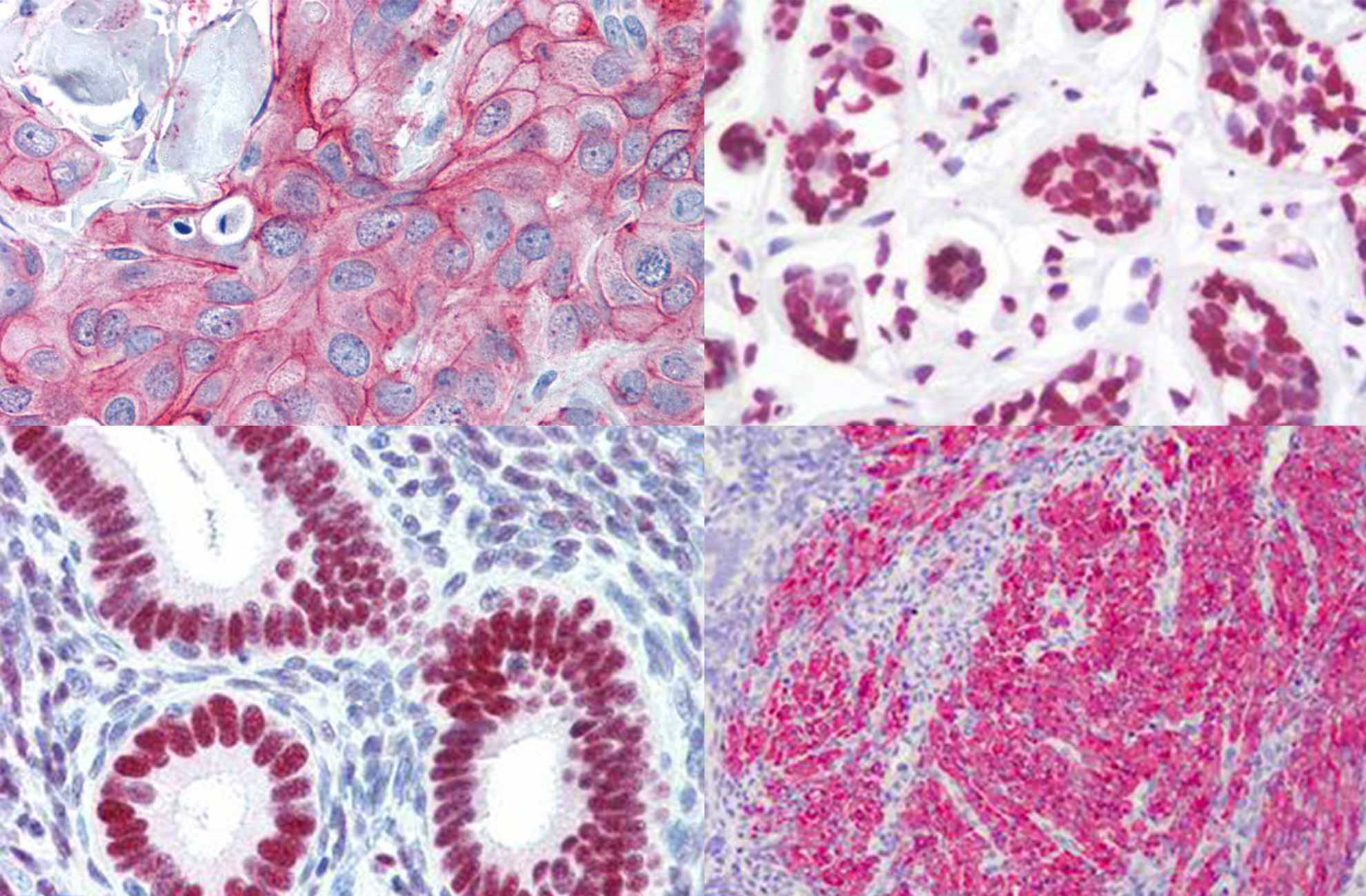
High quality PD-L1 recombinant antibody for IHC assay
GeneTex has worked with independent labs to validate the quality of its PD-L1 recombinant rabbit monoclonal antibodies (GTX636033 and GTX635975). The data demonstrates the antibody’s superior signal-to-noise ratio.

 Find products for your research
Find products for your research