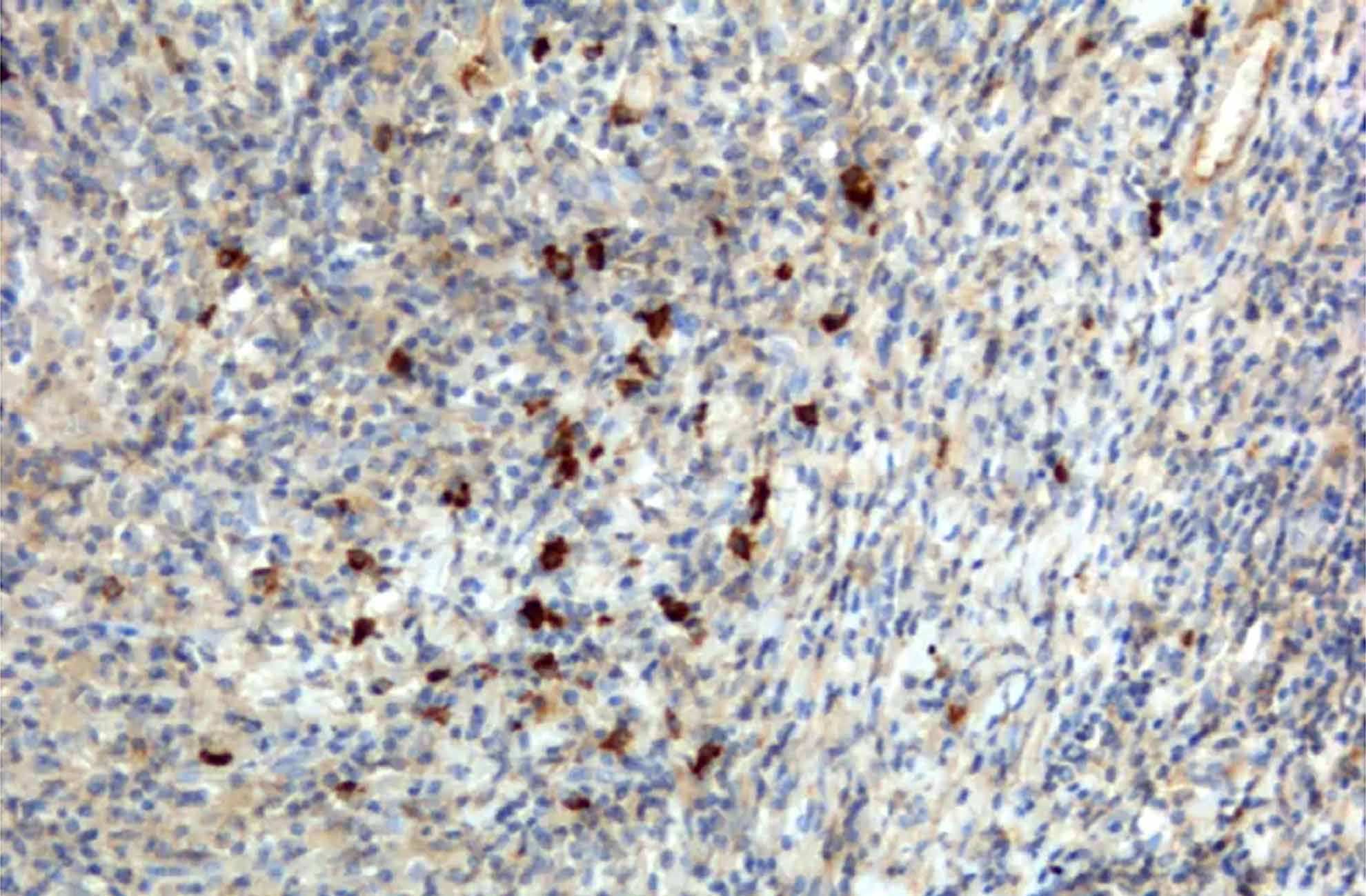
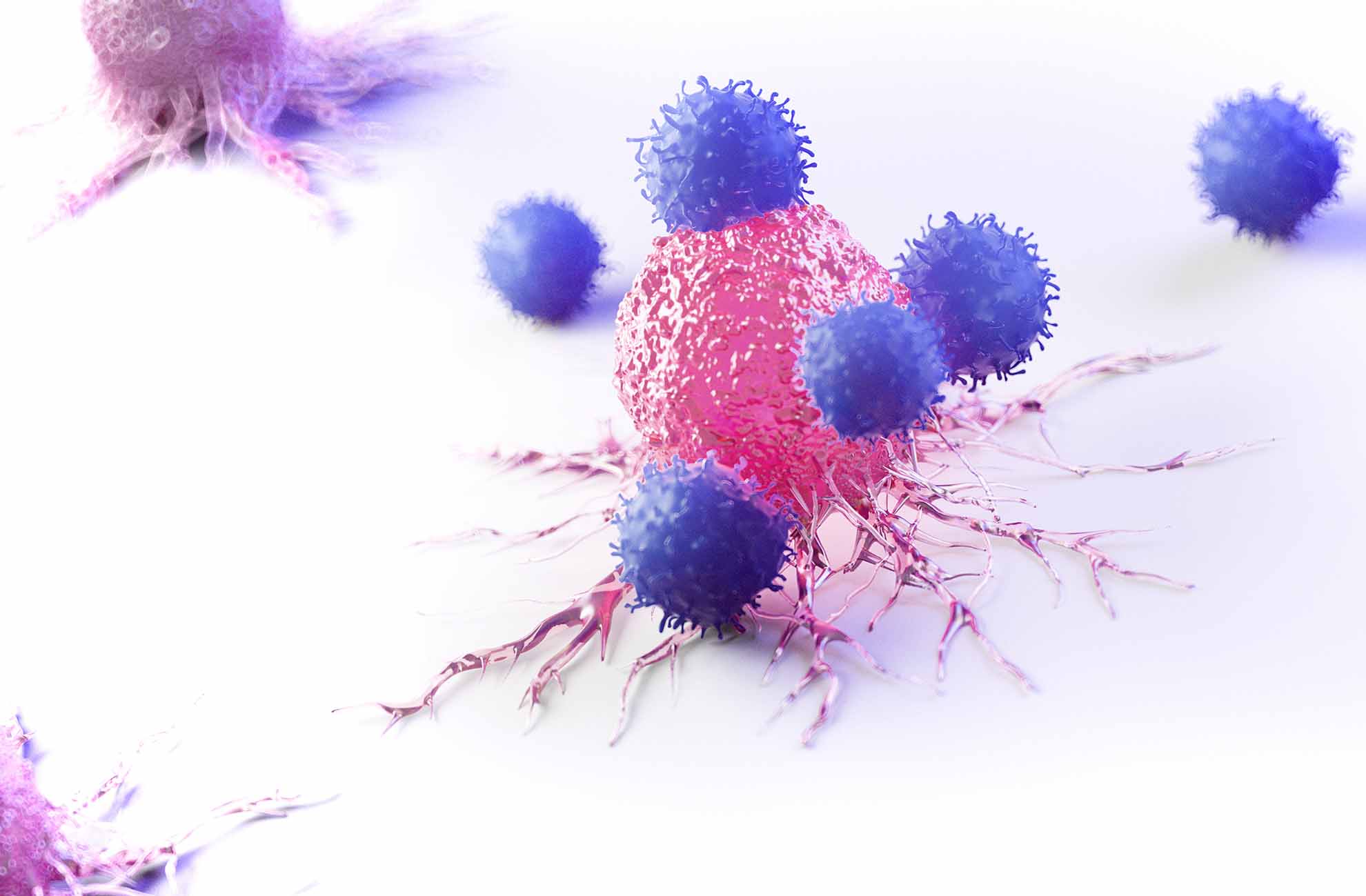
Unleash the therapeutic potential of NK cells
To support innovation in the ever-evolving landscape of immuno-oncology, BPS Bioscience offers a suite of NK Cell research solutions from ready-to-use primary cells and expansion kits to functional bioassays.